U2-2 - Hydrocarbons Flashcards
(36 cards)
Hydrocarbon
A compound which contains only hydrogen and carbon.
A group of compounds with the same general formula and similar chemical properties.
Homologous series
Homologous series:
A group of _________ with the same general formula and similar chemical properties.
Homologous series:
A group of compounds with the same general formula and similar chemical properties.
Homologous series:
A group of compounds with the _________ and similar chemical properties.
Homologous series:
A group of compounds with the same general formula and similar chemical properties.
Homologous series:
A group of compounds with the same general formula and _________.
Homologous series:
A group of compounds with the same general formula and similar chemical properties.
All alkane names end in …
-ane.
Alkanes are hydrocarbons in which the C atoms are joined by _______ carbon-to-carbon bonds.
Alkanes are hydrocarbons in which the C atoms are joined by single carbon-to-carbon bonds.

How many bonds does a carbon atom make?
Four
(because valency of carbon is four)

Name this hydrocarbon

Methane
Name this hydrocarbon

Ethane
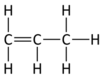
Name this hydrocarbon

Propane
Name this hydrocarbon

Butane
Name this hydrocarbon

Pentane
Name this hydrocarbon
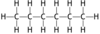
Hexane
Name this hydrocarbon

Heptane
Name this hydrocarbon
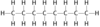
Octane
General formula for alkanes
CnH2n+2
(Twice as many Hs as Cs, plus two more)
Alkene molecules contain one …
double carbon-to-carbon bond, C=C.
All alkene names end in …
-ene.
What is missing from the name?

Butene
But-1-ene
Must show location of the double bond

Isomers
Compounds which have the same molecular formula but different structural formulae.
General formula for alkenes
CnH2n
(twice as many Hs as Cs)
Compounds which have the same molecular formula but different structural formulae.
Isomers
Cycloalkanes have ___ structures.
ring