Unit 1 Flashcards
(26 cards)
What is fermentation?
Fermentation is the conversion of rotting fruit or grain into alcohol solutions using yeast!
Pyruvate -> Acetaldehyde -> ethanol

Who was Dr. Louis Pasteur and what did he believe?
- Scientist that developed pasteurization to prevent bacteria from contaminating the yeat cultures used for making wine
- He believed that the ability of yeast to make alcohol required the ‘life force’ of yeast
Who was Dr. Justus von Liebig and what did he believe?
- Founder of organic chemistry who developed lab-based teaching.
- He was the father of fertilizier and developed boullion cubes for soup
- He believed that the ability of yeart to make alcohol did not require the ‘life force’ or yeast, rather that it is due to the chemistry
Who is Dr. Eduard Buchner?
- Chemist who was the founder of biochemistry
- He recieved the Nobel prize in 1907 for his discovery of cell-free fermentation
- He is also credited with proposing that these “enzymes” (molecules that mediate the physiological functions of cells) help to speed up reactions…basically these “enzymes” are catalysts
What was Dr. Eduard Buchner’s hypothesis, experimental design, and conclusion for the cell-free fermentation experiment?
- Hypothesis: live yeast are not required for alcohol to be produced (somewhat similar to Dr. Justus von Liebig)
- Experimental design:
- Generate a cell-free yeast extract
- Add the substrates (the food that the cell free yeast use to make the alcohol): glucose, fructose, maltose
- Monitor the products: CO2, ethanol, and energy
- Conclusion
- CO2 was produced by the cell-free yeast extract
- Thus, live yeast are not required in order for alcohol to be produced
What are catalysts?
Biomolecules that increase the rate of biochemical reactions dramatically
Where are catalysts found?
All living cells
What reactions are catalysts responsible for?
- Aerobic respiration
- Fermentation
- Nitrogen metabolism
- Energy conversion
- Programmed cell death
What are two natural catalyst examples that can be found in living cells?
Proteins and ribonucleic acid (RNA)
What are the six key biochemical principles?
- There is a hierarchical organization of biochemical processes within cells, organisms, and ecosystems that underlie the chemical basis of life on Earth
- DNA is the chemical basis of heredity and encodes RNA and protein which mediate biochemical processes in cells (The Central Dogma)
- The function of most biomolecules is determined by their structure (structure helps determine function: you can’t hammer something with a piece of paper)
- Biological processes follow universal laws and thermodynamic principles
- Life depends on water because of it’s properties and roles
- Biological membranes are selective hydrophobic barriers that define aqueous compartments where biochemical reactions take place
What is the organizational hierarchy of biochemistry in increasing complexity?

What does 97% of the weight of most organisms consist of?
Hydrogen, oxygen, carbon, nitrogen, phosphorus, and sulfur…most is hydrogen and oxygen presented as H2O (humans are 70% water)
What is the chemical nature of life on earth based on?
Carbon
What are unpaired electrons used for?
Unpaired electrons are used to make bonds between atoms, which lead to the creation of molecules like water, ammonia, carbon dioxide, carbonic acid
What is unique about carbon?
- Carbon has a unique ability to form up to four stable covalent bonds because of its four unpaired electrons
- This means that a chain of carbon atoms can serve as a backbone for the assembly of a variety of organic molecules
List the 7 most common carbon bonds

Discuss tetrahedron molecular geometry aka carbon single bonds
Four single bonds to a carbon atom are arranged in a tetrahedron as in methane CH4
Rotation can occur about the C-C bond

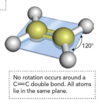
Discuss carbon double bond molecular geometry
- Arranged in a plane with double-bonded carbon atoms C=C such as ethylene (C2H4), all the atoms are in the same plane and the bond
- NO rotation about the C=C bond
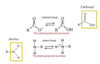
What are the 6 chemical/functional groups found in many biomolecules?
- Methyl -> only has 1 protonation state
- Phosphoryl, Carboxyl, Amino, Hydroxyl, Sulfhydryl -> all have different protonation sites but in the pics, only one is shown

Define protonation
The addition of a proton (H+) to an atom, molecule, or ion so that they can participate in chemical reactions
What are the four major classes of biomolecules?
- Amino acids
- Nucleotides
- Simple sugars
- Fatty acids

What are amino acids?
- Nitrogen-containing molecules that function primarily as the building blocks of protein
- Amino acids covalently link into linear chains to form polypeptides
- Each amino acid differ from each other by the side chain attached at the central carbon
- There are 20 different amino acids
What are nucleotides? What do they consist of? What are examples of them?
- Includes the nucleic acids, DNA and RNA (oligomers)
- Nucleotides consist of
- Nitrogenous base
- Five-membered sugar
- 1-3 phosphate groups
- Examples
- Cytosine
- ATP
- cAMP
- NAD+
What are simple sugars? What are some examples of them?
- Basically, simple sugars are carbohydrates that contain C, H, and O atoms ONLY
- They have a 2:1 ratio of hydrogen atoms to oxygen atoms
- They include
- Monosaccharides
- Disaccharies


