Vocab Ch15-16 Flashcards
(20 cards)
Miscible
If two liquids can dissolve into each other with all properties
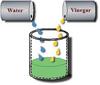
Immiscible
Liquids insoluble in another

Saturated Solution
Most amount of solute for given amount of solvent at a specific temp/pressure

Super Saturated Solution
More solute than can dissolve in the amount of solvent

Unsaturated Solutions
Less solute the max amount for the solvent to dissolve

Concentrated Solution
Large amount of solute

Dilute Solution
Small amount of Solute

Suspension
Where a solute is dispersed in a solvent to form a phase

Molarity
Number of moles of solute dissolved in 1 L of solution

Colloid
Homogeneous, noncrystalline/large/ultramicroscopic particles dispersed in another that cannot be seperated

Insoluble
Incapable of being dissolved

Emulsion
Fine dispersion of minute droplets of a liquid in another to allow it to become soluble

Heterogeneous
Substances involving different phases

Homogeneous
Containing only 1 phase

Solvent
Able to dissolve other substances, liquid which solute is dissolved in

Solvation
Interaction of solute within a solvent, stabilizes solute in solution

Solubility
Property of S.O.M to dissolve into another S.O.M

Solute
Dissolved in solvent

Solution
Liquid mixture where minor component solute is uniformly distributed in solvent

Concentration
Amount of solute dissolved in a given solvent



