8. Drugs Used in the Treatment of Cancer Flashcards
What are 7 causes of cancer?
Causes of cancer
- Genetic factors - eg. defects in genes BRCA1 or BRCA2 and breast cancer
- Ionising radiation - eg. acute leukaemia and thyroid cancer
- Chemical factors (carcinogens) - e.g. tobacco smoke and lung cancer; asbestos and mesothelioma
- Ultraviolet light and skin cancer
- Viruses - e.g. hepatitis B virus and hepatocellular cancer; human papilloma virus and cervical cancer; HIV and Kaposi’s sarcoma; Epstein Barr virus and Burkitt’s lymphoma
- Diets high in red meat and/or processed meat and colorectal cancer in some genetically susceptible individuals (diets high in vegetables, fruit and fibre decrease colorectal cancer)
- Helicobacter pylori and stomach cancer
What are the 3 main ways of treating cancer?
Cancer Treatments
The three major ways of treating cancer are:
- surgical removal of the tumour
- irradiation (radiotherapy)
- drug therapy (often called chemotherapy)
What is neoadjuvant chemotherapy?
What is adjuvant chemotherapy?
Neoadjuvant chemotherapy = chemotherapy given before surgery or radiation to reduce the size of the tumour
Adjuvant chemotherapy = chemotherapy given after surgery or radiation to improve survival
What are the Major classes of cancer drugs/chemotherapeutic agents?
(AHAHA)
Drugs Used to Treat Cancer
- Alkylating agents
- Hormone antagonists
- Antimetabolites
- HER2 inhibitors
- Aromatase inhibitors
Be aware of newer treatment options such as immune checkpoint inhibitors and CAR T-Cell Therapy
How do Alkylating agents work to treat cancer?
Example?
Drugs Used to Treat Cancer - Alkylating Agents
- Alkylating agents form covalent bonds across the strands of DNA to produce intrastrand links and interstrand cross links - attach primarily to nitrogen at position 7 (N7) in guanine
- This inhibits transcription, modifies DNA structure, inhibits DNA synthesis and inhibits cell division
- Alkylating agents can also exert marked effects on normal cells
- Example = Cyclophosphamide
- Cyclophosphamide is a pro-drug and is converted to its active metabolites by the cytochrome P450 system

How do Antimetabolites work to treat cancer?
Example?
Drugs Used to Treat Cancer - Antimetabolites
- Folates act as coenzymes for the synthesis of factors such as purine nucleotides which are essential for DNA synthesis and cell division
- In order to act as coenzymes folates must be reduced to tetrahydrofolate (FH4) by the enzyme dihydrofolate reductase
- Methotrexate is an antimetabolite which inhibits the enzyme dihydrofolate reductase
- Methotrexate inhibits DNA synthesis and cell division
- Methotrexate can also exert effects on normal cells

Which cells are most susceptible to the effects of Alkylating agents & Antimetabolites?
Alkylating agents & Antimetabolites
- Many cancer cells reproduce at a faster rate than normal cells, and this makes them more sensitive to alkylating agents and antimetabolites
- However, some normal cells also reproduce rapidly and this makes them also more susceptible to alkylating agents and antimetabolites
- Cells which undergo continuous, rapid division include:
- Those in the bone marrow
- Hair follicles
- Epithelium of the GIT
What Adverse Events are associated with Alkylating Agents and Antimetabolites in the:
- Bone marrow?
- Gastrointestinal tract?
- Hair follicles?
- Fertility?
Alkylating Agents and Antimetabolites - Adverse Events
- Bone marrow - leukopenia, neutropenia, thrombocytopenia (associated with a higher risk of bleeding events), anaemia, severe immunosuppression and infections and malignancie
- Gastrointestinal tract - gastrointestinal haemorrhage, acute pancreatitis, colitis, enteritis, mucosal ulceration, stomatitis, diarrhoea, abdominal pain, nausea and vomiting (release of 5HT)
- Hair follicles - alopecia which may progress to baldness which is reversible, although occasionally the new hair may be different in texture or colour
- Fertility – may interfere with oogenesis and spermatogenesis and may cause sterility in both sexes, which may be irreversible in some patients
What are 5 types of cancers that Cyclophosphamide is used for?
Cyclophosphamide - used to treat a number of cancers including”
- Hodgkin’s and non-Hodgkin’s lymphomas
- Multiple myeloma
- Leukaemias
- Neuroblastoma
- Adenocarcinoma of the ovary
Used in combination with other anticancer agents
What are 6 types of cancers Methotrexate is used for?
Methotrexate - used to treat a number of cancers including:
- Breast cancer
- Gestational choriocarcinoma
- Cancers of the head and neck
- Osteogenic sarcoma
- Bronchogenic carcinoma
- Acute lymphoblastic (stem cell) leukaemias
Used in combination with other anticancer agents
How do Hormone Antagonists work?
Drugs Used to Treat Cancer - Hormone Antagonists
- Cancers arising in hormone dependent tissues may have hormone receptors on the malignant cells which can stimulate growth
- many cancers of the breast are oestrogen dependent
- many cancers of the prostrate are androgen (testosterone) dependent
- Several drugs used in the treatment of breast cancer and prostate cancer inhibit the effects of these hormones
What is Tamoxifen and how does it work?
Is it a prodrug? What is its active metabolite?
Hormone Antagonists - Tamoxifen
- Tamoxifen is a selective oestrogen receptor modulator (SERM)
- It blocks oestrogen receptors in the breast, but stimulates oestrogen receptors in the uterus
- It is a prodrug and is converted to its active metabolite endoxifen by CYP2D6
- By blocking oestrogen receptors in the breast tamoxifen prevents oestrogen from binding to the oestrogen receptors
- This reduces gene transcription and inhibits tumour growth

Why should patients on tamoxifen have their CYP2D6 status measured?
Hormone Antagonists - Tamoxifen
- It is a prodrug and is converted to its active metabolite endoxifen by CYP2D6
- About 7-10% of the European white population lack/have reduced levels of CYP2D6 and are poor metabolisers of tamoxifen
- Patients on tamoxifen should have their CYP2D6 status measured
What are the adverse side effects of Tamoxifen?
Tamoxifen - Adverse effects
- Nausea, vomiting, light headedness, skinrash and occasionally fluid retention and alopecia
- Menopausal symptoms such as hot flushes
- Endometrial changes including hyperplasia, endometrial polyps and cancer of the uterus (tamoxifen stimulates oestrogen receptors in the uterus)
What are Aromatase Inhibitors?
2 examples?
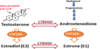
How are oestrogens primarily produced in post-menopausal women?
What is Aromatase?
Drugs Used to Treat Cancer - Aromatase Inhibitors
- Examples:
- anastrozole and letrozole
- In postmenopausal women, oestrogens are produced primarily from the conversion of androgens into oestrogens by the enzyme aromatase in the adrenal cortex and peripheral tissues e.g. androstenedione is converted to oestrone and testosterone is converted to oestradiol
- Aromatase inhibitors inhibit the aromatase enzyme and reduce the conversion of androgens into oestrogens