3.10 - 3.12 Cell to cell communication and signalling Flashcards
(69 cards)
What is the difference between contact dependent or paracrine signalling?
- This is for short distance
- Contact dependent is where cells are in close contact, membrane to membrane
- paracrine is where there is an extracellular release of signal that acts only locally on neighbouring cells

What are the long distance types of cell signalling?
- Synaptic where neurons have an electrical signal along the axon (long distance) resulting in release of neurotransmitter across the synapse (short distance)
- Endocrine where there is a release of hormone into the bloodstream which acts widely throughout the body

What type of signalling occurs at a very short distance?
- Autocrine where cells can stimulate themselves if they have receptor for the ligand
- Groups of identical signalling cells (community) can reinforce signals, for example in development it ensures cells follow the same differentiation pathway
- Cancer cells use autocrine signals to stimulate their own survival and proliferation

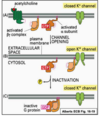
How can signalling occur in a gap junction?
- Allows direct communication between cytoplasm of adjacent cells by small intracellular signalling molecules such as Ca2+ or cAMP
- Allows neighbouring cells to coordinate responses to signal such as noradrenaline response in liver cells Cx32

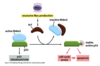
What are the two types of receptors?
- Cell surface receptors for a hydrophilic ligand that cannot cross the membrane. For example peptide growth factors bind cell surface receptor
- Intracellular for a hydrophobic or lipophillic ligand which can cross the membrane, for example steroids or small molecules diffuse across the membrane, bind intracellular/nuclear receptors

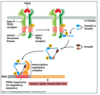
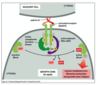
What is the basic cell signalling pathway?

How does the same signal (acetylcholine) cause different responses in different cells?
- Due to different receptor types
- Muscarinic (G protein) vs Nicotinic (ion channel) receptors
- Different intracellular mediators

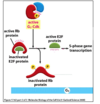
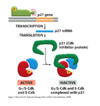
What are the characteristics of steroid hormones?
- They are transported in blood by carrier proteins (steroid-hydrophobic)
- Cross plasma membrane
- Bind intracellular receptors that have DNA binding domains (receptors are homo or heterodimers)

How are intracellular receptors activated?
- Receptors kept inactive as they have inhibitory proteins bound to them
- When ligand goes into cytosol it kicks off inhibitory protein, binds to the pocket and induces conformational changes



What are the 3 major classes of cell surface receptors?
- Ion channel couple receptors
- G-protein couple receptors (indirectly linked to enzymes)
- Enzyme coupled receptors

What are the two important classes of enzyme - coupled receptors?
- Receptor tyrosine kinases – have kinase activity and phosphorylate ‘Tyr’ on intracellular signal proteins
- Receptor serine/threonine kinases – have kinase activity and phosphorylate ‘Ser’ and ‘Thre’ on target proteins
What are the two classes of ligands for receptor tyrosine kinases?
Secreted growth factors and hormone
- Epidermal growth factor (EGF)
- Fibroblast growth factor (FGF)
- Platelet-derived growth factors (PDGF)
- Hepatocyte growth factor (HGF)
- Insulin, Insulin-like growth factor (IGF)
- Vascular endothelial growth factor (VEGF)
- Macrophage colony stimulating factor (M-CSF) • Neurotrophin (eg. nerve growth factor NGF)
Membrane-bound ligands
• Ephrins
What are the domains like for receptor tyrosine kinases?
- Single transmembrane domain
- Highly variable extracellular domains
- Similar intracellular domains (tyrosine kinase domains)

How do ephrins/eph receptors function together?
- very large family
- Can function in bidirectional signalling
- Ligand can signal back to the cell, main signal is via phosphorylation of Eph receptors, but ephrins often linked to the cytoskeleton so the signalling cell can receive a response as well
- Often functions in cell migration and axon guidance (attraction or repulsion cues)

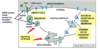
What is the signalling pathway for eph?
- Ephrins causes clustering of the eph receptors. Receptor on the migrating cell engages with ephrins and dimerises due to cross phosphorylation on a specific tyrosine
- A kinase binds resulting in phosphorylation of ephexin (GEF)
- Ephexin activates RhoA
- RhoA results in myosin-actin interactions and growth cone collapse
- No gene transcription, very rapid response
What conformational change do receptor tyrosine kinases undergo when a ligand binds?
- Ligand (dimer or multimer) binding causes receptors to dimerise (unlike G protein coupled receptors)
- Dimerisation causes cross phosphorylation of each receptor (autophosphorylation)

What does the phosphorylated receptor tyrosine kinase bind?
- The phosphorylated (activated) receptor binds other intracellular proteins via phospho-tyrosines
- Enzymes such as phospholipase Cgamma, phosphatidylinositol-3’-kinase, Src
- Docking proteins such as Grb2 which act as intermediary for enzyme to bind

What are Src Homology domains?
- Binding proteins have homologous phsopho-tyrosin binding domains
- SH2 binds activated phospho-tyrosines on receptor
- SH3 binds domains in other intracellular proteins
- Where that protein sits depends on its folding but once folded the SH3 domain interact with phosphotyrosine

What is the SH2 domain of the binding protein for receptor tyrosine kinase?
- it has two binding pockets, one for phosphotyrosine and one for amino acid side chain which is usually adjacent to phosphorylated tyrosine
- Phosphotyrosine same shape no matter what
- Plug and socket

How is Ras activated from receptor tyrosine kinase signalling?
- Activated RTK binds SH2 domain of Grb-2
- Grb-2 = docking protein (via SH3 domain) for guanine nucleotide exchange factors (GEFs) – eg Sos.
- SOS (GEF) activates Ras by exchange of GDP for GTP
- Ras now binds GTP and activates molecules downstream

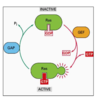
How does Ras function as an ON/OFF switch?
- Superfamily of monomeric GTPases such as Ran, Rab
- In the inactive state it binds GDP
- Activated by guanine exchange factors (GEFs) such as sos, where it exchanges GDP for GTP
- GTPase activating proteins (GAPs) result in increased hydrolysis of GTP
- Hyperactive Ras mutants are resistant to GAP which leads to cancer
Is the receptor tyrosine kinase and Ras active indefinitely?
- No receptor tyrosine kinase and Ras are active only for very short periods
- Action of phosphatases on receptors and GAPs (GTPase activating proteins) on Ras
- Therefore need signalling pathway to rapidly propagate these signals for proliferation and differentiation
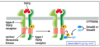
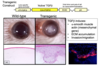
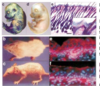
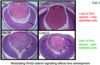
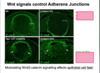
What is happening in this experiment?

- This experiment shows how short the activation of Ras is
- Ras is manipulated genetically and expressed in cells
- Linked GTP to a red fluorescent dye. When red comes in close contact with yellow there is resonance energy transfer
- When Ras binds GTP two fluoro molecules come close and you can measure the fluorescence coming off the red GTP
- Ras only active for 4-5 minutes