Atherosclerosis, Lipoproteins and Lipid-Lowering Agents Flashcards
(59 cards)
What is the main difference in the composition of LDLs and HDLs?
They have different apoproteins

What are dietary triglycerides and cholesterol packaged into once they are absorbed?
Chylomicrons
What are chylomicrons broken down into?
When hydrolysed by lipoprotein lipase the chylomicron releases TG core, free FAs and mono and diglycerides. Then it undergoes delipidation forming ….
Chylomicron remnants
Describe the exogenous pathway of lipid metabolism.
Exogenous pathway = transport and use of fats from diet.
- Fats in GI broken into cholesterol, fatty acids, mono and diglycerides.
- They become water soluble when combined with bile acids and can be absorbed in the duodenum.
- Virtually all TG absorbed but only 50% cholesterol
- Chylomicrons enter bloodstream via intestinal lymphatics and thoracic duct
- When in plasma, chylomicrons are hydrolysed by lipoprotein lipase releasing TG core, FAs and mono and diglycerides for energy production or storage.
- Residual chylomicron is delipidated forming chylomicron remnants, which are taken up by different tissues e.g. liver where they undergo lysomal degradation and are used for making new lipoproteins, cell membanes or excreted as bile salts.

Describe the endogenous patway of lipid metabolism.
- TGs, cholesterol, cholesterol ester and other lipoprotein particles are ransported in VLDL in bloodstream, where VLDL is delipidated using lipoprotein lipase = this is endogenous pathway of lipid metabolism.
- TG is removed from core and exchanged for cholesterol esters (from HDL mainly)
- Most VLDL is converted to LDL but the larger ones are lipolysed to IDL which is removed from plasma directly
- LDL formed then leaves the plasma in the forms of a number of subfractions: LDL I-IV
- Enterohepatic circulation provides a route of excretion of bile acids and cholesterol.

Are most circulating lipids endogenous or exogenous?
Endogenous
What forms of lipoprotein are the most atherogenic?Why?
- IDL and small dense LDL particles.
- They are absorbed by macrophages within the arterial wall to form lipid-rich foam cells, which is the initial stage in the pathogenesis of atherosclerotic plaques.
What are the enzymes used for lipolysis of VLDL and IDL.
Large VLDL = lipoprotein lipase
Small VLDL and IDL = hepatic lipase
Summarise reverse cholesterol transport.
- Cholesterol cannot be broken down within the body so is eliminated intact.
- It is transported via HDL from peripherl tissues -> liver to be excreted.
- HDL begins as a lipid-deficient precursor which changes into a lipid-rich lipoprotein which transfers cholesterol directly to liver or to other circulating lipoproteins to be transported to liver for elimination.

Why is HDL identifed as a protective factor against development of atherosclerosis?
HDL acts as a vehicle for the transport of cholesterol for elimination in the process of reverese cholesterol transport so it is said to be protective against atherosclerosis.
Define atherosclerosis.
Atherosclerosis is an inflammatory fibroproliferative disorder
What cells are recruited in the process of atherosclerosis?
Macrophages (which turn into foam cells) Fibroblasts Smooth muscle cells
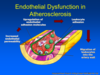
What must initially happen for the process of atherosclerosis to begin?
ENODOTHELIAL DYSFUNCTION precedes lesion formation. This includes:
- Increase in permeability of the endothelium
- Upregulation of leukocytes and cell adhesion molecules
- Leukocyte adhesion
- Migration of leukocytes into arterial wall
What is the name of the earliest recognisable lesion in atherosclerosis.?
Fatty streak = first visible sign of atherosclerosis to the naked eye
Happens early in human development but probably not all progress to atherosclerosis.
Describe what happens in fatty streak formation in atherosclerosis.
Aggregation of lipid-rich foam cells ( from macrophages and T-lymph) occurs inside the tunica intima. Later lesions include smooth muscle cells.
Steps involved:
- smooth-muscle migration
- T-cell activation
- foam cell formation
- platelet adherence and aggregation

Which cells are responsible for producing a protective fibrous cap over the fat core?
Smooth muscle cells lay down collagen fibres
What can happen as the atheroma grows larger?
Some of the foam cells die and rupture, releasing their toxic contents to form a lipid necrotic core
Describe how the complicated atherosclerotic plaque is formed in the advanced stages of atherosclerosis.
- Develops form a process of cell death and rupture of foam cells in the fatty streak.
- VSMCs migrate to the intima and lay down collagen fibres to form a protective fibrous cap over the lipid core.
- Fibrous cap separates the highly thrombogenic lipid-rich core from circulating platelets and other coagulation factors.
- Stable atherosclerotic plaques have a necrotic lipid core covered by a thick VSM-rich fibrous cap.
- Lesions expand at shoulders by continued leukocyte adhesion.

What is an unstable atherosclerotic plaque?
The fibrous cap thins and eventually ruptures, exposing the thrombogenic lipid core to the platelets and coagulation factors
This causes THROMBOSIS
NOTE: plaque erosion is also associated with hardening of the arteries, leading to weakening and thickening of the vessel wall leading to aneurysm and possible haemorrhage
NB: a stable atherosclerotic plaque is characterised by a necrotic core covereb by a thick VSM-rich fibrous cap.
What do complicated lesions often contain?
Calcium
What type of scanning is used to look at atherosclerotic plaques?
electron beam CT
This looks at the levels of calcium
If calcification predominates then the patient is at high risk of cardiovascular disease.
What is the significance of chylomicron remnants with regards to atherosclerosis? Which atherosclerosis pathway are they associated with?
- They are very good at getting into the tunica intima, therefore they are extremely atherogenic
- Can cause atherosclerosis in the same way as LDL but….
- Elevated levels of remnant cholesterol also causally associated with low-grade inflammation and IHD
- Elevated LDL is associated with IHD but not inflammation
- Indicating LDL doesn’t need an inflammatory component to cause atherosclerosis whereas remnant cholesterol does.

What are some characteristics of vulnerable plaques?
- Thin fibrous cap
- A core rich in lipid and macrophages
- Less evidence of smooth muscle proliferation
NB: SIZE of plaque does not indicate whether it is prone to rupture.
Vulnerable plaques are prone to rupture and ulceration, followed by rapid development of thrombi.

What are metalloproteinases associated with (released during plaque rupture)?
Breakdown of collagen