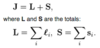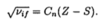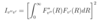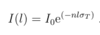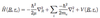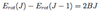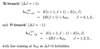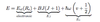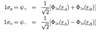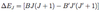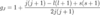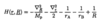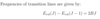Atomic Flashcards
What is meant by reduced mass?
- effective mass of a system once centre of mass motion is separated
- 1 particle orbiting the common centre of mass

What is meant by configuration, term and level? Give an example of each
- Configuration
- arrangement of the occupancy of number of e-s for a given orbital
- L
- Carbon: 1s2 2s2 2p2
- arrangement of the occupancy of number of e-s for a given orbital
- Term
- for a given configuration, arrangement of number of e-s are split into terms to include L and S for e-s outside closed shells & subshells
- L & S
- 1D
- for a given configuration, arrangement of number of e-s are split into terms to include L and S for e-s outside closed shells & subshells
- Level
- for a given term, arrangement of e-s are split into levels to include J which arises from spin-orbit interaction
- energy splitting from LS coupling
- levels are eigenstates of J2, Jz, L2, S2
- J, L & S
- 5D2
State Hund’s 3 rules
Lowest energy when
- For a given configuration, term with max. multiplicity 2S+1
- bigger S => lower E
- For a given multiplicity, term with largest L
- bigger L => lower E
- For a given term
- outer shell less than half-full
- lower J => lower E
- outer shell more than half-full
- bigger J => lower E
- half full
- no multiplet splitting (thought it was the lower J….?)
- outer shell less than half-full
What are the dipole allowed transitions for many electron atoms?
Consider rigorous and approximate selection rules.
Rigorous rules: (I changed te order so they’re now in order of strength)
- ΔJ = 0, ± 1 {J=0 => J’=0 Forbidden}
- ΔMj = 0, ± 1 (no effect unless there is a B or E field)
- Laporte rule - parity must change
Approximate rules:
- ΔS = 0
- ΔL = 0, ± 1 {L = 0 => L’ = 0 Forbidden} (Δl = ± 1 for 1 e-)
Strong transitions:
- atomic transitions are stronger the more dipole selection rules they fulfil
Dipole selection rule for Hydrogen.
Laporte rule - parity switch
Δl = ± 1
Δm = 0, ± 1
Δs = 0
If LS coupling is significant:
Δj = 0, ± 1 (but no j = 0 => j’ = 0)
List the approximate order of strength of the dipole transitions, including those that are forbidden.
- ΔS =/= 0 - Weakly forbidden (stronger transition than ΔL =/= 0, ±1)
- ΔL =/= 0, ±1 - Forbidden (stronger than J and Laporte rule transition)
- Laporte rule violation - strongly forbidden (weaker transition)
- J = 0 => J’ = 0 - Most strongly forbidden (weakest transition)
What is metastability?
- spontantenous decay from excited state i to all final states f is a forbidden dipole transition i.e. Aif ≈ 0
- any transition from i this unlikely to occur
- e- in metastable state i is trapped there
What do the quantum numbers l, s, j, ml, ms, mj represent and what values do they take?
These are the angular momentum quantum numbers.
- l = Orbital ang. mom; l = 0,1,2,…,n-1
- gives magnitude of orb. ang. mom => l(l+1)ħ2
- ml = orbital. mag. mom; -l,…,l
- gives component of orb. ang. mom => mlħ
- s = spin ang. mom; s = 1/2 (fermions), 0 (bosons)
- gives magnitude of spin ang. mom => s(s+1)ħ2
- ms = spin mag. mom; -s,…,s
- gives component of spin ang. mom => msħ
- j = total ang. mom; j = | l - s |,…, | l + s |
- gives total ang. mom. => j(j+1)ħ2
- mj = total mag. mom; -j,…,j
- gives component of total ang. mom => mjħ
- mj = total mag. mom; -j,…,j
- gives total ang. mom. => j(j+1)ħ2
Sketching the first few spherical harmonics.

Which quantum numbers are needed to completely specify the electrons state in hydrogen?
n, l and m
How do n, l and m arise from geometrical constraints on the wavefunction.
- n - arises from the need for the radial solution to converge
- => leads to energy quantisation, n is an integer.
- l - need for polar solution to converge, l is an integer.
- m - need for φ(…) = φ(…+2π), m must be an integer.
How do we find the radial probability density (measure of finding e- at distance from the nucleus)?
Give the number of peaks for the curve of a particular (n,l) state.
- total probability density for the wavefunction is split into radial and angular parts - don’t forget the spherical coordinates (Jacobian gives r2)
- radial probability density = integral of |Rnl(r)2| r2 from 0 to infinity
- spherical harmonics are normalised
- radial probability density = integral of |Rnl(r)2| r2 from 0 to infinity
- Can sketch the distribution for different quantum states in Hydrogen
- (n - l) peaks

Using the hydrogen radial distribution curves, what is meant by “electron penetration”” and what are the consequences for the order in which orbitals are filled?
- Electron penetration describes degree to which e-s in diff. (n,l) states penetrate region occupied by 1s electrons
- varies with l (and n, but less so)
- The more they penetrate this region, the less shielding and the more effective nuclear charge they feel.
- hence more tighly bound by the nucleus
- => have lower energy levels
- Since orbitals are filled in order of increasing energy, states with lower l are filled first
- For a given n, probability of finding e- near nucleus decreases with increasing l (further from nucleus) since centrifugal barrier pushes e- out so radius of sphere is greater
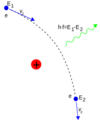
What is the Zeeman effect?
- splitting of spectral lines caused by energy shifts (arise from LS coupling) associated with magnetic dipole moment interacting with an external magnetic field is applied
Describe the weak field Zeeman effect.
- Normal zeeman:
- Atoms have zero net spin: closed shells/subshells
- Consider effect of only orbital angular momentum - μL
- Splitting only from interaction of L with B
- Anomalous zeeman:
- Net spin is non-zero
- Splitting from both L and S interaction with field
- => ΔE = μBBgJMJ (energy shift between the level splittings)
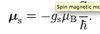
Describe the Paschen-Back case.
- B field is strong enough to uncouple L and S which then precess independently about the direction of the B field.
- Energy splitting now just the sum of the splitting due to L and S independently
- Can determine if field is strong by comparing it to LS splitting - if zeeman splitting is > the smallest LS splitting => strong field.
- ΔEL = μBBgLML
- gL = 1
- ΔES = μBBgSMS
- gS = 2
- ΔEL = μBBgLML
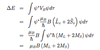
What is the Stark effect?
- Splitting of energy levels due to the application of an external E field.
What is the Quadratic Stark effect?
- atoms with no intrinsic dipole moment
- field polarises e- density inducing dipole moment p
- which is proportional to Eext
- so interaction energy VE = -p.Eext = -(1/2)αE2ext
- α = dipole polarisability
- hence varies quadratically with field
- interaction of dipole moment with E field => energy splitting
- energy shift given by eqn
- where A & B are level dependent constants
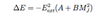
What is the linear stark effect?
- Atoms with intrinsic dipole moment (e.g. excited states of H & H-like atoms which have l-degeneracy)
- E field causes mixing of states with m =/=0
- applied E field in z direction connects states with Δl = ±1 & Δml = 0
- due to electric dipole selection rules
- applied E field in z direction connects states with Δl = ±1 & Δml = 0
- States with m=0 are centre on x-y plane but E in z direction
- E field pulls e-s to left or right and causes a mixing eigenstates of s and p states which then experience a +ve or -ve energy shift of ΔE
- Perturbing potential does not act on states where e- distributions are concentrated in x-y plane
- eigenstates of H = H0 + eEextz are also eigenstates of H0 so no work in mixing wavefns => energy is linear in Eext
- exctied state behaves as if it had electric dipole of magnetic 3ea0

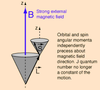
Describe the Stern-Gerlach experiment
- demonstrated the quantisation of e- spin in 2 orientations
- silver atoms beam in the s state placed in non-uniform B field
- atoms experience a force along B field direction
- Since mag. moment of atoms can classically point in any direction => spread expected?
- 2 beams in opp directions observed
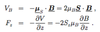
Linear stark energy diagram
- ψ+ ( sum of states) has e- density shifted towards -z, so mag. dipole is direction of E => ΔE < 0
- ψ- (diff. of states) has e- density shifted towards +z, so dipole antiparallel to E => ΔE > 0
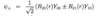
What is the quantum defect and how does it vary with the quantum numbers n and l?
- Quantifies departure from Hydrogenic behaviour - correction term to n.
- Alkali atom has single optically active (valence) electron
- further out it is (higher l), the smaller the effective nuclear charge it feels (due to inner e-s & their shielding effects)
- and the closer to a hydrogenic e- it is
- further out it is (higher l), the smaller the effective nuclear charge it feels (due to inner e-s & their shielding effects)
- Quantum defect decreases with l and n, although more slowly with n.
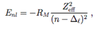
What is jj coupling?
- high Z atoms where spin-orbit interactions >> e-e interaction ( spin-spin or orbit-orbit interactions)
- individual orbital angular momenum combines with corresponding individual e- spin to from individual total angular momentum
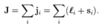
How to calculate the quantum defect
- Note in the original quantum defect formula, the energy represents the energy above the ground state
- => this is equivalent to the energy of the particular (n,l) state subtracting the ionisation energy (ground state energy)
- Replace the E in the formula with Enl with (Enl - Eion)
- Eobserved = Eion + Enl(outer electron)
- => Eobs - Eion/hc = -RMZeff2/neff2
- Zeff = 1 for alkali metal atoms