Batteries Flashcards
(61 cards)
The definition of a battery
A device that converts chemical energy into electrical energy
Which type of battery cells are rechargeable and which aren’t?
Primary cell are not rechargeable Secondary cells are rechargable
Why are secondary cell batteries called “storage batteries”?
Secondary cells do not produce electrical energy. They store it in chemical form
Each cell in a Lead-acid battery produces how much voltage?
2.1V
The 2 main purposes for an aircraft battery
- To provide backup emergency electrical power 2. To provide power for starting an engine
Identify
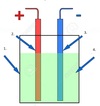
- Casing
- Annode
- Cathode
- Electrolyte
Identify

- Casing
- Positive terminal
- Negative terminal
- Cel divider
- Positive electrode (lead dioxide)
- Negative electrode (lead)
- Sulfuric acid and water
When a primary cell is fully charged, the potential differnce between the two electrodes is approximately
1.5V
In a closed circuit, as the transfer of electorns from the postive electrode to the negative electrode continues through the electrolyte, what eventally happens to the negative electrode in a primary cell battery?
It dissovles in the electrolyte
Oxidizing is the process of
loosing electrons
Identify (carbon-zinc)

- Metal cap
- Carbon rod
- Zinc Case
- Manganese Dioxide
- Ammonium chloride
- Metal bottom
The annodic, cathodic and electrolytic materials for an Alkaline battery
- zinc annode
- Manganese dioxide cathode
- Potassium hydroxide electrolyte
How can the composition of the electrolyte and electrodes and the chemical energy inside the electrolyte be restored?
Passing a charging current throught the battery in the reverse direction of discharge
The capacity of a battery cell is the measure of
How much current the battery can provide for a specific period of time
What must the charging voltage be while recharging a secondary cell battery?
Greater than the potential differnce between the electrodes
Capacity of a battery is measured in
Ampere hours
The determining factor of the capacity of a battery
Surface area of the electrodes (plates)
A cell with a capacity of 80 Ah should produce
80 amps for 1 hour
or
40 amps for 2 hours
or
8 amps for 10 hours
The 3 ways that battery cells can be connected in
- Serries
- Parallel
- Series-parallel
The total voltage in a battery where the cells are connected in series
The sum of the individual cell voltages
Desribe the total voltage and battery capacity of multiple battery cells arranged in series
the voltage will be the sum of the individual cells but the capacity will remain the same
Desribe the total voltage and battery capacity of multiple battery cells arranged in parallel
The total voltage will that of 1 cell, but the capacity will be the sum of the individual cell capacities
If two 12V, 80Ah batteries are connected in series, how will they act?
As a battery with 24 V output, 80 Ah
If two 12V, 80Ah batteries are connected in parallel, how will they act?
They will act as one battery with 12V out put, at 160Ah


