Biofilms Flashcards
(8 cards)
How do biofilms form? P.aeruginosa, different shapes? (problems for liquid flow in medical device?)
Cell-cell and cell-surface contacts, quorum sensing signals, sticky EPS (extracellular polymeric substrate):
P.aeruginosa can form mushroom shapes in low flow
And form filamentous streamers in high flow (can block tubes, reduces flow rate)
Biofilm heterogeneity induced by nutrient gradients? 3 ways?
Metabolic substrate gradient (like oxygen in aerobes) concentration lowers towards centre
Metabolic product gradient (e.g. methane in methanogens) increases concentration towards centre
Metabolic intermediate reaches high concentration part-way through biofilm then decreases (e.g. denitrifying bacteria use up waste nitrite?).

3 different genetic mechanisms for creating heterogeneity in bacterial biofilms?
1) Adaptive gene expression (different genes, induced by different environments/gradient) (e.g. facultative anaerobes in oxygen concentration gradient)
2) Mutation/genetic variation and natural selection (e.g. non-mucoid mutants of Strep.pneumoniae –> better attachment, less protection)
3) Stochastic/random gene expression (can lead to persister cells in infection, more variety [independent of environment] –> more chance of resistance/resilience)
(“bistable switch” formation, e.g. EPS on or off in Bacillus subtilis, SinI anti-repressor sometimes expressed, de-represses Eps operon (from SinR repressor)
Why are biofilm infections difficult to treat (and even diagnose)? examples?
Difficult to treat: Resistant to phagocytosis due to large size.
- *Resistant to many antibiotic drugs** (due to variability and slow growth)
- *Persister cells** (due to stochastic gene switching, and other genetic variation)
Diagnosis is difficult because they are localised infections.
Examples: P.aeruginosa in CF patients
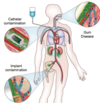
Infective endocarditis (Strep. vegetations on damaged endothelium, layers of fibrin and platelets interspersed!)
Medical device infections
4 diagnostic criteria for biofilm infection diagnosis?
- Pathogenic bacteria surface associated (invasive investigation)
- Direct examination reveals bacteria clustered encased in matrix
- Localised infection
- Abx resistant infection (when planktonic cells would be sensitive)
Three mechanisms by which Biofilms could be resistant to ABx?
Slime matrix (EPS) forms barrier or dilutes Abx
Slow growing, slowly metabolising cells less susceptible (e.g. to beta lactams)
Genetic heterogeneity –> resistant persisters left behind
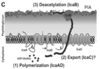
3 Stages of staphylococcal biofilm lifecycle: key molecules in each stage?
- Attachment (MSCRAMMS and autolysin AtlE)
- Maturation (PIA, polysaccharide intercellular adhesion, deacetylated by icaB. –>positive charge, interacts with -ve [lipo]teichoic acids)
- Dispersal: Agr quorum sensing promotes dispersal! Increased detachment factors PSM (phenol-soluble-modulins) and decreased attachment factors (EPS and autolysin AtlE)
What is Disperin B?
PIA degrading enzyme found in wound dressings used to discourage biofilm formation.