Carbohydrates Flashcards
(10 cards)
Describe the 2 smallest and simplest monosaccharides
The smallest and simplest monosaccharides are trioses (3 carbon sugars/C3)
- 2 trioses:

How is the [α] of light rotated by a molecule determined?
What is expected for enantiomers, diastereomers, meso compounds?
- Enantiomers rotate plane polarised light to the same magnitude but in opposite directions
- Diastereomers are unpredictable
- Meso cannot rotate light

How do I determine whether a sugar is D or L?
What is this difference at one stereocenter called?
- Penultimate carbon
- The furthest stereocenter (from C1) is usually the penultimate one
- If the OH is to the right, it is D; if left, it is L
- Epimer: a difference at just one stereocenter

What does the following nomenclature indiciate?
- # Deoxy - no OH at specified location
- # Amino - an NH2 replaces the OH at specified location
- # N-acetylamino - an NHCOCH3 replaces the OH at specified location
- Glucose –> glucuronic acid - CH2OH is replaced by COOH
- Furanose
- Pyranose
- # Deoxy - no OH at specified location
- # Amino - an NH2 replaces the OH at specified location
- # N-acetylamino - an NHCOCH3 replaces the OH at specified location
- Glucose –> glucuronic acid - CH2OH is replaced by COOH
- 5 membered sugar: furanose
- 6 membered sugar: pyranose
What happens when monosaccharide hydroxyl and carbonyl groups react?
- They form a hemiacetal ring (intramolecular)

Mechanism for acid-catalysed hemiacetal formation?

Mechanism for base-catalysed hemiacetal formation?

How can this sugar be drawn as a Haworth projection?
- How do I determine D and L in this form?

C6 (CH2OH group) is up for D, down for L

What is the anomeric carbon?
-
Anomeric carbon: C1, the most oxidised carbon
- Exception: D-fructose, where C1 is swapped with C2
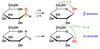
What are anomers?
- Created during the formation of the Haworth projection; the carbonyl group is sp2 and can freely rotate, so when the penultimate OH is attacked it creates 2 stereoisomers
- Note: if an OH group other than the penultimate carbon is involved, assignment is not the same