Chapter 5 Flashcards
(38 cards)
1
Q
thermodynamics
A
the study of energy and its transformations
2
Q
thermochemistry
A
- the study of the relation between chemical reactions and changes in energy
- chemical rxns: energy transferred as heat
3
Q
heat(q)
A
- the energy transferred between objects because of a difference in their temperatures
- not a state function
4
Q
thermal equilibrium
A
a condition in which temperature is the same throughout a material and no further energy transfer occurs
5
Q
work(w)
A
- the energy required to move(cause motion) an object through a given distance
- work is a form of energy
- work = Force x distance
- not a state function
- w = -PΔV
- work done by the system leads to an increase in the system’s volume AND transfers energy to the surroundings
6
Q
potential energy (PE)
A
- the energy stored in an object because of its position
- on a moecular level:
- chemical bonds and differential electric charges cause interactions btwn particles that give rise to PE
- temperature governs motion
7
Q
state function
A
- a property based solely on a current chemical and/or physical state
- it is independent of the path followed to acquire PE
- ΔH and ΔE are state functions (also T,V,etc)
- depends only on difference btwn initial and final states
- it is independent of the path followed to acquire PE
8
Q
kinetic energy(KE)
A
- KE depends on mass and velocity
- velocity depends on temp too so KE depends on temp
- the energy of an object in motion due to its mass and speed
- KE = 1/2(m)(u)2
- u=speed
- KE on a molcular level
- as temp increases, avg. KE increases
9
Q
thermal energy
A
- the KE associated with the total random motion of molecules
- thermal energy is proportional to temp
- thermal energy depends on # of particles in a sample
- more molcules = more themal energy if temp is constant
10
Q
electrostatic potential energy (Eel)
A
- the energy a particle has b/c of its electrostatic charge and its position w/ respect to another particle
- Eel is proportional to (Q1 x Q2)/d
- d = distance btwn two particles
- Q1 and Q2 = charge of each particle
- postive Eel = particles repel
- negative Eel = particles attract
- A lower electrostatic PE (a more negative Eel) indicates greater stability
- total energy always = KE due to random motion of particles + PE due to particle arrangment
11
Q
energy(E)
A
- the ability to do work and/or transfer heat
- the energy given off/absorbed during a rxn is equal to the difference in the energy of the reactants and the products
- positive = absorbed?
- negative = released?
- energy ca be used to do work, transfer heat
12
Q
system
A
- the specific part of the universe we are studying
- often a chemical rxn or physical process
13
Q
surroundings
A
- everything that is not part of the system
14
Q
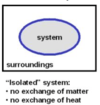
isolated system
A
- a system that exchanges neither energy nor matter with the surroundings
- a thermos of hot soup with the lid screwed on tightly
15
Q
closed system
A
- a system that exchanges energy, but NOT matter with the surroundings
- a cup of hot soup with a lid
- heat goes out
- a cup of hot soup with a lid
- most real systems we deal with are closed b/c they are easier to model quantitatively

16
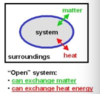
Q
open system
A
- a system that exchanges both energy and matter with the surrooundings
- an open cup of hot soup
- matter goes out as steam
- matter goes in as crackers cheese and pepper
- heat goes out
- an open cup of hot soup
- cells, organisms, and Earth are all open
17
Q
exothermic process
A
- energy flows from system to surroundings
- q is negative
18
Q
endothermic
A
- energy flows from surroundings into system
- q is positive
19
Q
internal energy(E)
A
- E = KE of all components system + PE of system
- not possible to determine exact values
- ΔE= Efinal - Einitial
- a state function b/c ΔE depends only on the initial and final states….how the change occurs in the system doesn’t matter
- doing work on a system adds to its internal energy
- total increase of the internal energy of a closed system
- ΔE = q+w
20
Q
first law of thermodynamics
A
- essentially the law of conservation of energy
- The energy (ΔE=q+w) gained or lost by a system must equal the energy gained or lost by the surroundings
- ΔEsys+ΔEsurr=0
- when work is done by a system on its surroundings, internal energy of system decreases
- pressure-volume work(P-V work):
- the work associated with the expansion or compression of a gas
- when the pressure on a system remains constant but the volume of the system changes
- a hot air balloon- heating the balloon causes volume to increase even though atmospheric pressure remains constant
21
Q
calorie(cal)
A
the amount of energy necessary to raise the temp of 1 gram of water by 1˚C
22
Q
joule(J)
A
- the SI unit of energy
- 4.184 J = 1 cal
23
Q
enthalpy change (ΔH)
A
- the heat absorbed by an endothermic process or given off by an exothermic process occurring at constant pressure
- ΔH = qp = ΔE + PΔV
- qp = enthalpy change at constant pressure
- ΔH = qp = ΔE + PΔV
- ΔH<0 when energy flows out of a system and q<0
- ΔH>0 when energy flows into a system and q>0
24
Q
enthalpy(H)
A
- ΔH is defined as heat transferred at constant pressure
- ΔH>0 = endothermic
- ΔH<0 = exothermic
- the sum of the internal energy and the pressure-volume product
- H = E + PV
- difficult to calculate so we use ΔH
- H = E + PV
- unit is J or J/g or J/mol
- ΔH and ΔE represent changes in a state function of a system
25
difference between ΔE and ΔH
* ΔE includes *all* the energy(heat and work) exchanged the sys with the surr
* ΔE = q + w
* ΔH is *only* q, the heat, exchanged at constant pressure
* ΔH = qp
* ΔE and ΔH are very similar with constant volumes
26
molar heat capacity (cp)
* the quantity of energy required to raise the temp of 1 mole of a substance by 1˚C at constant pressure
* q = ncpΔT
* ΔT is in Celsius
* units = J/mol˚C
27
specific heat(cs)
* the quantity of energy required to raise the temp of 1 gram of a substane by 1˚C at constant pressure
* units = J/g˚C
28
molar enthalpy of fusion(ΔHfus)
* the energy required to convert 1 mole of a solid substance at its melting point into the liquid state
* Ex: the energy absorved as snow melts
* q = nΔHfus
29
molar enthalpy of vaporization (ΔHvap)
* the energy required to convert 1 mole of a liquid substance at its boiling point to the gas/vapor state
* q = nΔHvap
30
calorimetry
* the measurement of the quantity of energy transferred during a physical change or chemical process
* **calorimeter:** device used to measure absorption or release of energy by a physical change/chem process
* assume a **closed system**
* heat from sys = heat to surr
* qsys + qcal =0
31
enthalpy of reaction(ΔHrxn)
* used to quantify the (heat) energy absorbed or given off by a chemical reaction under constant pressure
* * aka heat of reaction
* ΔHrxn\<0 when exothermic aka heat is released
* exiting system and going into surroundings
* ΔHrxn\>0 when endothermic aka heat is absorbed
* entering system from the surroundings
32
thermochemical equation
the chemical equation of a reaction that includes the change in enthalpy that accompanies that reaction
33
heat capacity(Cp)
* the quantity of energy needed to raise the temperature of an object by 1˚C at constant pressure
* referred to as a calorimeter constant (Ccal)
* this value is unique to every calorimeter so units are NEVER J/g˚C or J/mol˚C
* units=J/˚C
* qcalorimeter=CcalorimeterΔT
* we can then use qcal to calculate quantities of energy produced
* qcal = -ΔHrxn
34
Hess's law
* the principle that the enthalpy or reaction(ΔHrxn) for a reaction that is the sum of two or more reactions is equal to the sum of the ΔHrxn values of the constituent reactions
* ΔH is a state function, so we can...
* 1) multiply the coefficients by a common factor
* 2) reverse the rxn if we flip the sign of ΔH
* Hess's law works b/c enthalpy is a state function
* for a particular set of reactants and products, the enthalpy change is the same regardless of how many steps the rxn took
35
standard enthalpy of formation(ΔH˚f)
* the enthalphy change of a **formation rxn**
* **formation rxn:** when 1 mole of a substance is formed from its constituent elemtns in their standard states
* aka [standard] heat of formation
* formation reaction DOES NOT EQUAL real life synthesis
36
standard conditions+states
* indicated by the ˚ in ΔH˚f, **standard** **conditions** are 1 atm, usually 25˚C, and 1M concentration
* **standard states:** the most stable form of a substance under standard conditions
* a pure element in its most stable form under standard conditions has ΔH˚f = 0
37
standard enthalpy of reaction(ΔH˚rxn)
* the energy associated with a rxn that takes place under standard conditions
* aka standard heat of rxn
38
phase changes



