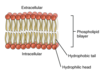
Exam 1 Flashcards
(144 cards)
anatomy
study of structure

systematic anatomy
study of structures that make up a discrete body system
physiology
study of function
standard anatomical position
standing facing forward, feet hip-width distance apart palms our, proper posture

anterior/ ventral
front
posterior/dorsal
back
superior
above/ towards head
inferior
medial
toward the midline
lateral
away from the midline
proximal
closer to the trunk
distal
farther from the trunk
superficial
more external
deep
more internal
Name the cross-sections

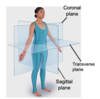
midsagittal
along midline
parasagittal
not along midline
cellular composition
made up of 1 or more cells
organization
display order and hierarchy
metabolism
internal chemical reactions
produces waster –> excretion
responsiveness
ability to sense and react to stimuli
movement
at all levels of organization
Developlment
change in form or function over an organism’s lifetimes
reproduction
formation of a new organism