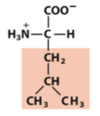
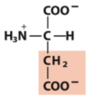
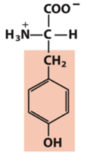
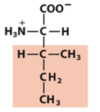
Exam 1 Flashcards
(267 cards)

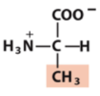
ethyl

phenyl
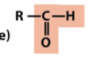
carbonyl (aldehyde)
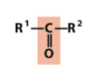
carbonyl (ketone)

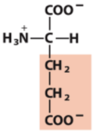
carboxylate

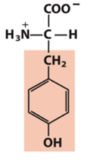
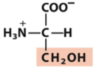
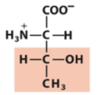
hydroxyl (alcohol)
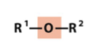
ether

ester

acetyl

anhydride
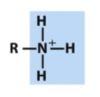
amino (protonated)

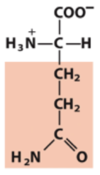
amido
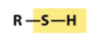
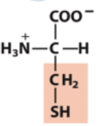
sulfhydryl

disulfide

thioester

phosphoryl
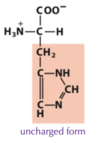
R/S configuration rules
- Compare the atomic number (Z) of the atoms directly attached to the stereocenter; the group having the atom of higher atomic number receives higher priority.
- If there is a tie, consider the atoms at distance 2 from the stereocenter—compare the atoms bonded to the one directly attached to the stereocenter; at the earliest difference, the group containing the atom of higher atomic number receives higher priority.
Br > Cl > S > –OCH3 > –OH > –NH2 > –COOH > –CHO > –CH2OH > –CH3 > –H

water hydrogen bonding
- Water molecules attracted to one another due to partial charge differences between O and H - Hydrogen bond
- strong noncovalent attraction
- Almost tetrahedral shape allows for water molecules to H-bond with 4 other water molecules -> ice

hydrogen bonds
readily form between any electronegative atom (the hydrogen acceptor) and a hydrogen atom covalently bonded to another electronegative atom (the hydrogen donor)
water polarity
- Due to it’s polarity and ability to hydrogen bond, water can act as a polar solvent to dissolve charged or polar molecules by hydrating them
- Since many biomolecules are either charged or polar they easily dissolve in water (hydrophilic) and are soluble in the aqueous environment of the cell
- Water dissolves salts by weakening the electrostatic interactions between the ions that allow it to form a crystal lattice
- Solvation causes increase in entropy since ions are freed from crystalline structure thus delta G is negative (ΔG= ΔH-TΔS)
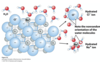
hydrophobicity
- When a non-polar solute is placed in water, the water molecules in the immediate vicinity of the non-polar molecule are constrained in their orientations.
- The result is an overall increase in entropy of the water ΔS > 0 since water becomes less restricted upon micelle/cluster formation.
*

ordered waters in binding interactions
Individually these are small energies, but collectively they make large contributions to biochemical processes.

acid/base property of water
water can ionize
ionization of water
In non pure water solutions, [H+] and [OH-] will change but together still must equal a Kw of 1 x 10-14 M2
Kw is basis for pH scale which tells concentration of H+ and therefore
OH- as well