Final Flashcards
(64 cards)
Acid ends in -ate
ending in -ic acid ex. chlorate -> chloric acid
Acid ends in -ite
ending in -ous acid ex. chlorite -> chlorous acid
Acid ends in -ide
ending in -ic acid prefix hydro- ex. chloride -> hydrochloric acid
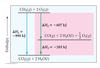
The one with the second fewest oxygens
ends in -ite
The one with the second most oxygens
ends in -ate
The one with the most oxygens
per_____ate
The one with the least oxygens
hypo_____ite
Cation
metal
Anion
nonmetal
Group 1A
Alkali Metals
Group 2A
Alkaline earth metals
Group 6A
Chalcogens
Group 7A
Halogens
Group 8A
Noble Gases
Beta rays (charge)
negative
Alpha rays (charge)
positive
Gama rays (charge)
none
Naming (order to name)
Cation first; Anion second
Intensive properties
inside material ex. density, boiling point, color
Extensive properties
depends on how much material there is ex. mass, volume, energy
Combination reactions
Two or more reactants combine to form a single compound.
Decomposition reactions
A single reactant breaks apart to form two or more substances.
Combustion in Air
Hydrocarbon + Oxygen -> Carbon dioxide and Water.
Formula Weight
is the sum of the atomic weights for the atoms in a chemical formula (once been reduced)