lecture 29: stem and iPS cells Flashcards
(41 cards)
What are the different types of stem cells?
- Embryonic stem cells
- isolate from the inner cell mass of the blastocyst
- origin: blastocyst of embryo
- strengths:
- pluripotent (3 germ layers)
- self-renewal and high replicative capacity
- weaknesses:
- immunological concerns
- subject to ethical debate
- potential for teratoma and teratocarcinoma
- currently no clinical trial data
- Adult SCs
- origin: bone marrow, circulation or resident tissue
- strengths:
- autologous
- clinical safety and efficacy data
- typically lineage commited
- weaknesses:
- limited number
- limited replicative capacity
- lineage restricted
- iPSCs
- origin: reprogramming of somatic cells
- strengths:
- totipotent (3 germ layers and trophoblast)
- autologous
- large reservoir of cells
- weaknesses
- potential for teratoma and teratocarcinoma
- no clinical data
What defines a stem cell?
- self-renewal - maintenance of ‘stemness’
- potency/potential - capacity for differentiation
- indefinite proliferation
- telomerase activity
- normal karyotpe maintained
- marker expression profiles
- embryoid body formation
- teratoma formation
- directed: neurons, cardiomyocutes, haematopoietic progenitors, insulin producing cells
What are differing potencies of stem cells?
- totipotent: fertilised oocyte and cells after first cleavage divisions; ability to form entire organism
- pluripotent: cells of the ICM of the blastocyst; ability to form all three germ layers but not the extraembryonic tissues; embryonic stem cells
- multipotent: mesenchymal stem cells which can form bone, cartilage and fat; ability to form multiple cell types; adult stem cells
What is embryo development?
- day 0: fertilisation
- fertilised egg (zygote)
- day 1: first cleavage
- day 2: 2 cell stage
- day 3 - 4: 4 cell stage, 8 cell uncompacted morula
- day 4: 8-cell compacted morula
- day 5: early blastocyst, trophectoderm, blastocoel, inner cell mass
- day 6 - 7: late-stage blastocyst, leaving zona pellucida
- day 8 - 9: implantation of the blastocyst: epiblast, hypoblast
What are the varying tissue lineages over the course of embryo development?
- fertilised egg ( day 0)
- blastocyst (5)
- trophoblast (6-7)
- cytotrophoblast (8-9)
- syncytioptrophoblast (12)
- cytotrophoblast (8-9)
- inner cell mass (6-7)
- hypoblast (8-9)
- extraembryonic endoderm (12)
- yolk sac (14)
- extraembryonic endoderm (12)
- epiblast (8-9)
- amniotic ectoderm (12)
- primitive ectoderm (12)
- embryonic ectoderm (15)
- primitive streak (14)
- embryonic endoderm (15)
- embryonic mesoderm (15)
- extraembryonic mesoderm (15)
- hypoblast (8-9)
- trophoblast (6-7)
What is the history of embryonic stem cells?
- establishment in culture of pluripotent cells from mouse embryo
- isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells
- isolation of a primate embryonic stem cell line
- embryonic stem cell lines derived from human blastocysts
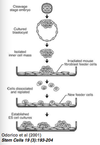
- Odorico et al (2001):
- cleavage stage embryo
- cultured blastocyst
- isolated inner cell mass
- cultured on irradiated mouse fibroblast feeder cells
- cells dissociated and repleted
- new feeder cells
- established ES cell cultures
What are embryonic stem cells?
- all human lines available are derived from excess embryos (IVF)
- process of isolation (immunosurgery, laser)
- many isolated onto mouse embryonic fibroblast (MEF) feeder layers, with (bovine) serum
- undefined conditions
- antigens found on stem cells
- disease transmission from use of animal products
- FDA will not approve use for transplantation
- late passage numbers available for study (adaptation with culture)
- enzymatic passaging protocols
- karyotypic instability
What controls self-renewal?
- sox 2
- oct 4
- nanog
- klf 4
- myc
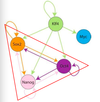
What is a model core ES cell regulatory circuitry?
What characterises embryonic stem cells?
- morphology
- transcription factor expression
- dependence on glycolytic metabolism and glutaminolysis
- long telomeres, high telomerase activity
What characterises pluripotency?
- chimera formation
- differentiation (in vitro, teratoma formation in vivo)

What is developmental potential/differentiation?
- stem cell division and differentiation
- symmetric division
- asymmetric division
- progenitor division
- terminal differentiation
- pluripotent cell → unipotent
- induction (environmental): growth factors; other factors
- commitment (cell autonomous): epigenetic; transcription factor networks
- patterning (environmental): positional information
What is the relationship between developmental potential and epigenetic status?
- totipotent zygote
- global DNA demethylation
- pluripotent
- e.g. ICM ES cells, EG cells, EC cells, mGS cells, iPS cells
- 2 active X chromosomes
- global repression of differentiation genes by Polycomb proteins
- promoter hypomethylation
- multipotent
- e.g. adult stem cells (partially reprogrammed cells?)
- x inactivation
- repression of lineage-specifc genes by polycomb proteins
- promoter hypermethylation
- unipotent
- e.g. differentiatied cell types
- x-inactivation
- derepression of polycomb silenced lineage genes
- promoter hypermethylation
What are epigenetic mechanisms?
- affected by these factors and processes:
- development (in utero, childhood)
- environmental chemicals
- drugs/pharmaceuticals
- ageing
- diet
- DNA methylation
- methyl group (an epigenetic factor found in some dietary sources) can tag DNA and activate or repress genes
- histones are proteins around which DNA can wind for compaction and gene regulation
- histone modification
- the binding of epigenetic factors to histone “tails” alters the extent to which DNA is wrapped around histones and the availability of genes in the DNA to be activated
- health endpoints
- cancer
- autoimmune disease
- mental disorders
- diabetes
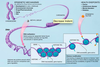
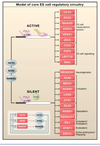
How does the epigenetic response to extrinsic signals occur?
- occurs through a network of transcription factors
- active genes
- active chromatin
- pluripotent cells
- differentiatied cells
- master regulators e.g. oct 4, NANOG, Sox 2 etc
- auxillary factors: myc etc
- lineage specific genes upon differentiation
- poised genes
- bivalent chromatin
- pluripotent cells
- genes of early response to differentiation,
- silent genes
- silent chromatin
- pluripotent cells
- differentiated cells
- lineage specific genes in ES cells
- master regulators upon differentiation
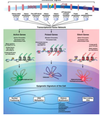
What are adult stem cells?
- drive the renewal of all adult tissues
- divide continuously to produce new cells that undergo a robust differentiation programme
- limited repair and regeneration
- in culture:
- highly refractory to expansion and long-term culture
- difficult to isolate homogenous populations

When were adult stem cells discovered?
- 1950s
- bone marrow: two different stem cell populations
- haematopoietic stem cells → red and white blood cells, platelets
- bone marrow stromal cells → bone, cartilage, fat, stroma

How are adult stem cells isolated?
- haematopoietic stem cells
- bone marrow and its peripheral blood
- placenta and umbilical cord
- mesenchymal stem cells
- bone marrow of the iliac crest or femoral head
- adipose tissue
- identified by surface marker expression
Where have ASCs been found?
- many different tissues: blood/bone marrow, heart, fat, epidermis, retina, dental pulp
- bone marrow stem cells (MSCs)
- widely used for transplantation (HLA compatibility)
- adipose tissue stem cells
- differentiated towards functional cardiomyocytes, osteoblasts, haematopoietic and neural cells
- cord blood stem cells
- transplantation is an accepted curative therapy and non-malignant inherited diseases
- useful for child transplantations, hampered in adults by low cell dose
- disadvantages:
- cells move away from the transplantation site
- cell integration is not significant/cell death
What is reprogramming?
- reversing the differentiation process

What are methods of reprogramming?
- somatic cell nuclear transfer (SCNT)
- SCNT using an embryo at mitosis
- altered nuclear transfer (ANT)
- fusion of skin cells with hESCs
- induced pluripotent stem cells, or iPSCs

What is somatic cell nuclear transfer?
- first attempts at nuclear reprogramming were in frog eggs, later followed by attempts in mammals
- early studies were followed by several successful cloning experiments using enucleated oocytes and donor nuclei (even ICM nuclei) in a number of livestock species
- key conclusions from successful experiments were:
- egg cytoplasm, but not zygotic cytoplasm was permissive to reprogramming
- eggs must be enucleated to maintain normal ploidy in the developing embryos
- clones have been generated from various foetal and adult cell types, with varying degrees of success
- cloned embryos ≠ fertilised embryos
- poor blastocyst rates
- most cloned embryos die during gestation
- developmental defects
- genome wide gene expression abnormalities
- epigenetic inheritance likely the principle barrier
*

What is altered nuclear transfer (ANT)?
- eliminate the capacity for a cloned blastocyst to implant normally
- experiments confirmed that this approach is feasible in mouse
- don’t yet know whether the human Cdx2 gene has a similar function in trophoblast development
- scientifically: negates any relationship between the ICM and TE












