Neoplasia Flashcards
(55 cards)
Define Neoplasia
- NOT the same as malignant
- Neoplasia is a disorder of cell growth (from an array of causes) that is triggered by one or more mutations affecting a single cell and its clonal progeny
- Monoclonal - almost always malignant
- Polyclonal - not always malignant
- There are benign and malignant form of neoplasia

Define Benign tumors
- tumor is considered benign when its gross & microscopic appearances are considered relatively innocent, implying that it will remain localized, will not spread to other sites and is amenable to local surgical resections

Define malignant tumors
- latin = crab
- Tumor is considered malignant when its gross & microscopic appearances (and locations) indicates that it will not remain localized, and has or will spread to other sites, making cure from local excision likely
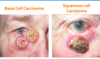
Nomenclature - Carcinoma
- Modifiers may refine the epithelial source
- Adenocarcinoma means glandular epithelium (ex. breast cancer)
- Bronchogenic carcinoma - epithelium of airway passages
- Tumors from connective & muscle tissue - hard (bone), soft (stromas)
Nomenclature - Sarcoma
- More deadly then carcinoma
- Modifiers may refine source
- Rhabdomyosarcoma - striated muscle
- Leimyosarcoma - smooth muscle
Nomenclature - Tumors from neural cells
-
Glioma (benign)
- Oligodendroglioma
- Astrocytoma
-
Glioblastoma
- Glioblastoma multiforme
- Retinoblastoma
Nomenclature - Tumors from brain coverings
-
Meningioma - brain covering
- same term used for benign & malignant with addition of invasive, if malignant
Nomenclature - Tumors from blood vessles and blood cells
- Leukemia - derived from granulocytic, nucleated blood cells
- Lymphomas - derived from lymphocytes or their precursors
- Multiple myeloma - malignancy of plasma cells
- Hemangioma/Lymphangioma - benign tumor of blood/lymphatic vessels (image)
- Hemangioblastoma/Lymphangiosarcoma - malignant tumor of blood/lymphatic vessels

Nomenclature of benign vs malignant CT tumors
-
Benign tumors from CT usually end in -oma
- Leiomymoa (smooth muscle)
- Rhabdomyoma (skeletal m)
- Chondroma (cartilage)
- Fibroma (Soft CT - fibroblasts)
- Osteoma (bone)
-
Malignant tumors from connective & muscle tissues usually end in -sarcoma
- Leiomysarcoma
- Rhobdomysarcoma
- Chondrosarcoma
- Fibrosarcoma
- Osteosarcoma
- Exception is malignant form melanoma (CT melanocytes). - think of melanosarcoma, b/c that is how it behaves
- Benign form is nevus
What does this image show?

- Iris leiomyoma
- The only smooth muscle in the iris is the sphincter (NOT Dilator, myoepithelium)
- Mass at the pupil
What does this image show?

- Diabetic lacy vacuolization of the iris that increases deposition of glycogen in epithelia but NOT smooth muscle.
- Since dilator muscle is the myo component of epithelium, it is affected. The sphincter, being true smooth muscle, is not. Hence diabetic do not dilate well but constrict just fine
Epithelial Dysplasia
- consists of an expansion of immature cells (such as ectoderm), with a corresponding decrease in the number and location of mature cells
- Dysplasia is often indicative of early neoplastic process.
- The term dysplasia is typically used when the cellular abnormality is restricted to the originating tissue, as in the case of an early, in-situ neoplasma that occupies less than the full thickness of the epithelium
- Nothing to do with hip displasia (development anomalies)

Conjunctival Intraepithelial Neoplasia (CIN)
- Carcinoma in-situ - full replacement of epithelium
- If the process has invaded the basement membrane, it is considered to be squamous cell carcinoma

Differentiation vs Anaplasia
- Differentiation describes the degree to which neoplastic tissues resemble the full differentiated cells from which they derive and retain their differentiated function (well differentiated, looks like normal tissue)
- Anaplasia poor cellular differentiation
- The more differentiated the tissue = less malignant potential it has
-
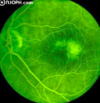
Primary retinal tumor caused by mutation of a tumor suppressor gene.
- Avg 18 mo but as late as 12 yrs
- Retinoblastoma
Name the degrees of differentiation of retinoblastoma mimic photoreceptor differentiation

What does this image show?

- Poorly-differentiated (anaplastic) Retinoblastoma
Degree of De-differentiated Ortho vs. Parakeratosis
- In several neoplastic skin conditions (most of which are not malignant), the epithelium is turning over too rapidly (cell balance is loss)
- As a result, certain differentiative stages are sacrificed
- Perakeratosis - absence granular layer (stratum granulosum) and retention of nuclei in the stratum corneum.
- Hyperkeratosis - thicker keratin on surface, but the granular cell layer is retained and the keratinized cells have lost their nuclei

What are the cellular characteristics of Malignancy
- Pleomorphism
- Prominent nucleoli
- Coaruse clumping of chromatin
- Increased mitotic index/Tripolar mitoses
- Loss of polarity & loss of contact inhibition
What does this image show?

Cellular characteristic of malignancy - pleomorphism

Cellular Characteristics of malignancy - Prominent nucleoli
What does this image show?

Cellular characteristics of malignancy - coarse clumping of chromatin
aka hyperchromaticity
What does this image show?

- Cellular characteristic malignancy
- Left increased mitotic index
- Tripolar mitoses
What does this image show?



- Note pleomorphism, prominent nucleoili, increased mitotic index, loss of of polarity and loss of contact inhibition, but minimal hyperchomaticity