Tissue Repair Flashcards
(32 cards)
Define Regeneration & Repair
- Regeneration - primarily a feature of “labile” (ex. continuously dividing) epithelia. This process produces not only structural repair but re-establishment of normal structure & function
- Repair a process that re-establishes structural integrity but without re-establishing normal structure or function of the affected tissues. This process is seen with “stable” tissues and “permanent” tissues and usually produces a scar
Define Labile Tissue
include primarily bone marrow derived cells & body surface, continously replicating epithelia (ex. skin, cornea, lining of gut). These tissues can readily regenerate after injury as long as their pool of stem is preserved
Define Stable tissues
- Include cells that are inactive (G0 phase of the cell cycle) and have minimal mitotic and differentiative capacity. This mostly occurs after injury.
- Cells including parenchyma of most solid organs (kidney, pancreas) and many simple epithelia (corneal & trabecular endothelium, RPE, vascular endothelium, fibroblasts and smooth muscle cells.
- Some have stem cell populations (corneal endothelium, TM) but even when injured, they cannot restore total cell numbers to normal

Define Permanent Tissue
- Terminally differentiated and non-proliferative cell populations in adult life.
- These include neurons, cardiac, and skeletal muscle cells. These injuries are irreversible and repair with scarring.
Define Regeneration regarding the eye

A superficial corneal abrasion, or skin abrasion, or abrasion of the intestinal lining can regenerate, meaning it can fully restore normal structure & function if the stem cell population is intact. In the cornea, this usually involves a population of stem cells at the limbus.
If the underlying basement membrane is largely intact, cell signaling can lead to cell sliding from residual cells. These cells proliferate, detach and slide and cover the defect and then differentiate to create a fully anchored basal layer. Limbal stem cells then repopulate the total cell mass

Interference with epithelial wound healing
- Main factors that inhibit epithelial cell wound healing must be considered in clinical care
- DM
- Application of topical steroids
- Alcohol abuse
- Malnutrition
- Imunosuppression
Recurrent Corneal Erosion
- In some cases (especially abrasions caused by fingernails and the edge of a sheet of paper), regeneration of the basal epithelial adhesion complex may remain incomplete
- These pt may remove entire sheets of epithelium with their first opening of their eyes in the morning. Focal areas of faulty basement membrane may appear opaque (EBMD)
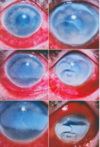
Recurrent corneal erosion / EBMD

- In recurrent erosion or epithelial basement membrane disease (EBMD) there is faulty basal cell adherence to substrate. And interference with hemidesmosome formation. Mostly due to weak integrin linkages
- Focal areas of faulty basement membrane may appear opaque (EBMD) due to basement membrane reduplication over time
Loss of the stem cell population can preclude epithelial wound healing
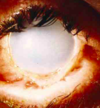
This eye suffered an alkali burn 9 months ago. Corneal epithelium renewal was not possible because the lye not only killed off the corneal epithelium but also the limbal stem cells.
Autologous, amniotic membrane limbal stem cell transplants
In cases where some stem cells remain, a biopsy of those cells can be grown out on amniotic membrane and then transplanted to the limbal region
These are before & after photos of several cases of amniotic membrane stem cell transplants

Regeneration after Labile epithelial displacement
In some cases of trauma or surgery, epithelial cells can become displaced into underlying stroma & survive. The surface epithelium heals but the buried epithelial cells still turn on their regeneration machinery
Spherical epithelial inclusion cyst over soft tissue

EIC formation
- A surface epithelial cell is driven below surface & survives
- The cell proliferates and each new cell directs its basal surface and basement membrane toward the surrounding stroma
- The sphere is closed
- The epithelium now has regained its desired state. a basal surface on a basement membrane, touching stroma and an apical surface abutting a different environment
- The cell of the cyst wall now feel the same as if they were again at the surface regeneration, from their perspective, is now complete
- They resume making whatever they normally make (e.g. fluid, keratin, etc), which fills the cyst cavity & expands (ex. they have fully regenerated but in the wrong place)

Hemi-spherical conjunctival inclusion cyst over sclera (clear vs yellow opaque)
- Forms ballon hemisphere - epithelial inclusion cyst beneath surface
- Clear = enter eye through limbus, red reflex, sheared zonules and dislocated lens (left image) close to limbus
- Yellow opaque = top right, globin cells make mucus
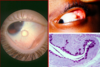
Epidermal inclusion cyst
- cells of the cyst wall return to making keratin

Regeneration in stable tissues
(Which organ has the most amazing regeneration?)
-
Some regeneration are limited in organs that have stable tissue
- Pancreas, adrenal, thyroid and lung all have limited regenerative ability
- Compensatory regeneration can occur in kidney. Surgical removal of one kidney leads to both hypertrophy & hyperplasia of ducts cells
- Replicating islet cells in limited regeneration of pancreas
-
The most amazing regeneration of a stable organ is LIVER
- In this process, cells of donor liver are first “primed” with human growth factor (HGF) and then epithelial growth factor (EGF) - induced proliferation. In humans, removal of 90% of the liver can be corrected through regeneration by remaining hepatocytes.
- Priming occurs through having hepatic reticuloendothelial cells (Kupffer cells) release IL-6, making quiescent hepatocytes capable of receiving and responding to signals from growth factors. What makes proliferation stop remains uncertain.
- Mimics acient myth
Wound Healing & Repair
- Phases
- Hemostasis
- Inflammation
- Proliferation
- Remodeling

Platelet Plug
- Injury
- Blood clotting first few mins of injury
- Platelet begins to stick to injured site
- Platelets are activated causing them to take amorphorous shape & release chemical signals to promote clotting
- This activates fibrin which acts as glue to bind platelets together
- Clot plug blood vessels to slow/preventing further bleeding
- Rupture of blood vessels brings platelets into contact with surrounding stromal collagen, platelets bind directly to collagen to create platelet plug
- adhesion is further strengthened by von willebrand factor (vWF) that helps form addition links between the platelets and collagen
- This also triggers the “clotting cascade”

Define Extrinsic & Intrinsic pathway (Hemostasis)
- The Intrinsic pathway (aka the contact activation pathway) is activated by damage directly to the blood vessel and the exposure of collagen to the circulating platelets within blood
- The extrinsic pathway (aka the tissue factor pathway) is activated by many things including damage directly to the blood vessel, tissue damage & hypoxia
- Ultimately, the 2 key players in the common pathway are thrombin & fibrin.
- Fibrinogen (leading to fibrin) is what traps the platelets, and is therefore clotting factor number 1.
- Prothrombin (leading to thrombin) is the 2nd most important becaue it activates just about everything

Describe PT & INR
-
Prothrombin time (PT)
- PT evaluates the efficacy of the extrinsic pathway of coagulation
- The prothrombin time is the time it takes plasma to clot after addition of tissue factor (factor 3) plus calcium in excess to overcome the citrate initially added to blood samples to prevent clotting.
- The normal value varies by method but is usually around 12-13 seconds
-
International normalized ratio (INR)
- INR stands for international normalized ration. The INR was created to normalize PTTs to eliminate variability in PTTs reported by different labs
- The INR is the ratio of a patients prothrombin time to a normal (control) sample, raised to the power of the ISI value for the analytical system being used
- The ISI (International sensitivity index) value indicates how a particular commercial batch of tissue factor compares to an international reference tissue factor
- A normal INR is approximately 1.0. People taking the blood thinner warfarin typically have a target INR of 2.0 to 3.0.

Initiating Inflammation
- Vasospasm Immediatley after a blood vessel is breached, endothelial cells membranes release inflammatory factors like thromboxanes & prostaglandins that cause the vessel to spasm to prevent blood loss & collect inflammatory cells & factors in the area. As clot achieves hemostasis, vasospasm gives way to vasodilation
- Vasodilation: Vasodilation is produced by factors such as histamine released by platelets and other cells
- Histamine increased vascular permeability rendering the tissue edematous. this increases osmolarity drawing fluid into the area
- Increased permeability also facilitates entry of inflammatory cells

Inflammation - tissue injury caused by physical or chemical agent or pathogenic microorganism

Proliferation
- In this phase we see
- angiogenesis
- Collagen dispostion
- granulation tissue formation
- epithelialization
- wound contraction occur
- In wound contraction, myofibroblast decrease the size of wound by gripping the wound edges and contracting using a mechanism that resembles that in smooth muscle cells
- When the cells roles are close to complete, unneeded cells under go apoptosis
- TGF B is the most important cytokine stimulating synthesis and deposition of extracellular matrix protein fibrosis

Epithelialization
- To make way for the growth of epithelium, the clot (now a scab) has to be dissolved by plasmin (derived from plasminogen via tissue plasminogen activator (t-PA)
- Granulation tissue stroma must be dissolved as well using MMPs, collagenase, gelatinase and stromelysin

Remodeling
- Remodeling is said to have begun when the amount of collagen being broken down by proinflammatory degradation and phagocytosis, equals the amount of new collagen being produced through proliferation
- collagen will reach approx 20% of its tensile strength after 3 weeks, increasing to 80% by 12th week
- The max scar strength is 80% of that unwounded skin
- Since activity at the wound site is reduced, the scar loses its red appearance as blood vessels that are no longer need are removed by apoptosis
- During remodeling, collagen is realigned along tension lines, and cells that are no longer needed are removed by programmed cell death, or apoptosis
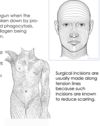
- Surgical incisions are usually made along tension lines because such incisions are known to reduce scarring