OChem (104) Flashcards
(53 cards)
Methane
CH4
BP -164 degrees C
(gas at room temp)
Ethane
CH3CH<span>3</span>
BP -89 degrees C
gas at room temp
Propane
CH3CH2CH3
BP -44 degrees C
gas at room temp
Butane
CH3CH2CH2CH3
BP -0.5 degrees C
(gas at room temp)
Pentane
CH3CH2CH2CH2CH3
BP 36 degrees C
(96 degrees F)
liquid at room temp
HEXANE
CH3CH2CH2CH2CH2CH3
BP 68 degrees C
liquid at room temp
Heptane
CH3CH2CH2CH2CH2CH2CH3
BP 98 degrees C
liquid at room temp
OCTANE
CH3CH2CH2CH2CH2CH2CH2CH3
BP 125 degrees C
liquid at room temp
nonane
CH3CH2CH2CH2CH2CH2CH2CH2CH3
BP 151 degrees C
liquid at room temp
Decane
CH3CH2CH2CH2CH2CH2CH2CH2CH2CH3
BP 174 degrees C
liquid at room temp
(alkane chains longer than decane are solids at room temp)
METHYL
CH3–
Methyl is a common alkyl group
Ethyl
CH3CH2–
Ethyl is a common alkyl group
Propyl
CH3CH2CH2–
Propyl is a common type of Alkyl group
Butyl
CH3CH2CH2CH2–
Butyl is a common type of alkyl group
isopropyl
isopropyl is a common alkyl group

tert-butyl
tert-butyl is a common type of alkyl group

Constitutional (structural) isomers
Molecules that have the same molecular formula, but differ in how the atoms are connected.
Have different properties.
Strucure of an alkyne functional group

cycloalkane are formed with…
single C–C bonds in a ring formation
Characteristics of an alkene?
- general formula CnH2n
- one of 4 types of hydrocarbons
- Contain at least one C–C DOUBLE bond, with sp2 hybridized carbons.
- Unsaturated, i.e. contain less H than an alkane with the same number of C atoms
- Rotation around the C–C double bond require breaking the π bond. Therefore, can form trans and cis geometric isomers.
Characteristics of an alkane?
- one of 4 types of hydrocarbons
- Saturated (every C has the max number of H)
- Single C–C bonds
- can be straight line or cyclo
- generally very reactive, produce much energy so often burned for fuel
What are the 4 types of hydrocarbons?
Alkane
Alkene
Alkyne
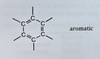
Aromatic
cycloalkane general formula
CnH2n
straight-line alkane general formula
CnH2n+2