Particle Physics Flashcards
(41 cards)
protons
positively charged particles found in the nucleus. not a fundamental particle. composed of 3 quarks. charge: +1, weight: 1
neutrons
uncharged particles found in the nucleus. not a fundamental particle. composed of three quarks. charge: 0, mass: 1
electrons
negatively charged particles orbiting nucleus. fundamental particle. charge: -1, mass: 0
specific charge
specific charge = charge (c) / mass (kg)
representing atoms

isotopes
atoms of the same element that contain different no. of neutrons. physical properties differ slightly.
forces acting on nucleons
At very short distances of less than 0.5fm, the strong force causes nucleons to repel one another strongly. there is also strong electrostatic repulsion.
at about 1.5fm (typical nucleus radius) the strong force becomes strongly attractive and holds the nucleons together, balancing the repulsive electrostatic force.
further from the centre, beyond 3fm, the strong force drops off rapidly. alotugh electrostatic repulsion has also decreased, the strong force cannot hold the nucleon in the nucleus.

alpha decay
the equivalent of a He nucleus being given off. an unstable nucleus, x, emits an alpha particle (2p + 2n).
easily stopped, sheet of paper. dangerous is swallowed.

beta - decay
gives off electron and antineutrino.

the strong force
- 1 of 4 fundamental forces of nature, the others being gravitational, electromagnetic, weak nuclear force
- provides attractive force between nucleons with a range of ~3fm
- overcomes the repulsive electrostatic force exerted by positively charged protons on each other
- at distances <0.5fm the strong force is repulsive and prevents the nucleus imploding/collapsing
gamma decay
electromagnetic radiation emitted from an unstable nucleus.
- gamma rad often occurs straight after α / β decay. the child nuclide formed often has excess energy whis is released by gamma emission.
- the transition isn’t perfect between stages in α decay. in β there’s only 1 jump
- no change occurs to nucleon numbers as a result
- gamma is pure energy
neutrinos
always emitted with β decay.
- β- decay results in antineutrino
- β+ decay results in a neutrino
- neutrinos are very difficult to detect as they have nerly 0 mass and no charge. they barely interact with matter. billions of these particles that have been emitted from the sun, sweep through our bodies every second.
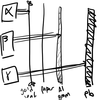
what stops the radiation types
α stopped by gold leaf.
β stopped by ~8mm Al
γ stopped by several cm of Pb
- why Pb? well. it’s the heaviest non-radioactive element. because of its large mass it hinders the radiation. smth even heavier would be better though it’s radioactive itself.
electricmagnetic radiation
radiation emitted by charged particles losing energy.
- e- decreasing in energy inside an atom (light)
- the biggest source of radiation available to us is the SUN
- e- losing kinetic energy when stopped by a solid material (X-rays)
the radiation consists of two linked electric and magnetic field waves which are
- at right angles to each other
- are in phase (peak together)
why are γ rays dangerous
γ rays are dangerous because their wavelength is about as big as a nucleus. they just pass right through, penetrating just about everything
the wave equation
wave speed = frequency * wavelength
photon
- electromagnetic radiation is emittd as short bursts
- each packet of waves is called a photon
- each photon contains a set amount of energy and is proportional to the frequency of the ER
photon energy EQ:
E = planck constant * freq.
antimatter
all particles of normal matter (p,n,e-) have a corresponding particle that:
- as the same mass as the normal particle
- has the opposite charge as the normal particle
- will undergo annihilation with the normal particle if they meet
antiparticles
- antiproton: neg. charged p
- positron: pos. charged e-
- the antineutrino produced in β- decay
- other particle properties are also reversed; uncharged antiparticles such as the antineutron
- two particles w/ same mass and opposite charges, not necessarily particle-antiparticle pair
- antimatter symbol: bar above letter
- certain man-made isotopes are made in order to provide a source of antimatter. eg. positrions are needed for PET scans
annihilation
- when a particle and its corresponding antiparticle meet together annihilation occurs
- all of their mass and kinetic energy is converted into two photons of equal freq. that move off in opp. directions

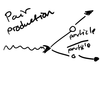
pair production
- opposite of annihilation
- the energy of one photon can be used to create a particle and its corresponding antiparticle
- the photon ceases to exist afterwards
electron volt
- the electron volt (eV) is a very small unit
- eV = 1.6 * 10^-19 J
- eV = kE gained by an e- when it is accelerated by a potential difference of 1V
particle rest energy
energy equivalent of mass -> E = mc^2
masses of subatomic particles quoted in energy terms in MeV.
early atom models
-
1800’s : Dalton’s model.
- each element its own specific atom w/ specific weight and indivisible.
- 1897: discovery of subatomic particles- e-
- the plum pudding model. positive body w/ negative dots in it
-
rutherford’s model
- mostly empty atom w/ a core: nucleus
- proved wrong because the attraction between - and + would be too strong and collapse on itself
- bohr’s model 1973
- neg. charged e- in circular orbits. planetary model. as e- goes up/down valence shells, quantum leap.