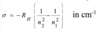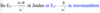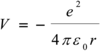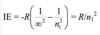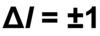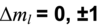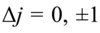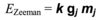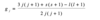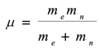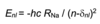Physical Flashcards
(83 cards)
Describe atomic spectroscopy
The study of the absorption and emission of photons by atoms. Experimental observation prompted the idea that energy is quantised.
Give the equation that governs which energies are possible for the hydrogen atom and explain all the terms
E is measured in joules
h is planck’s constant
c is the speed of light
Rh is the Rhydberg constant for the H atom
n is the principle quantum number

Describe what happens when an electron is excited to a higher energy state
The atom absorbs a specific amount of energy equal to the separation of the levels. (E2-E1 = hf = hv - h is planck’s constant and f is v (nu) is the frequency)
Describe what happens when an electron drops down to a lower energy state
The atom emits a specific amount of energy equal to the separation of the levels (E2-E1 = hf = hv - h is planck’s constant and f is v (nu) is the frequency)
State the equation for photon energy
Photon energy = hf = E2 - E1 = hv (should be a very small number) E2 is the higher energy f is v (nu) is the frequency
Describe absorption spectra
Absorption spectra measure the wavelengths of light that are absorbed by a sample, as the atoms take up energy and move to higher energy levels. All species start in their ground state
Describe how emission spectra are obtained
Energy is put into a sample of atoms to excite them to higher levels. The wavelengths of the light emitted when the atoms give up energy and drop back down is measured
Describe how energy is given to a sample of atoms in emission spectra
The sample is heated or an electrical discharge is applied
State the four components needed to carry out a typical absorption spectroscopic experiment
- a light source
- a sample
- a wavelength selecting element
- detector
Describe the light source needed in a typical absorption spectroscopic experiment
As there has to be a broad range of wavelengths produced, white light is used
Describe the sample needed in a typical absorption spectroscopic experiment
The sample normally has to be gaseous, which means it must be heated beforehand
Describe the wavelength selecting element needed in a typical absorption spectroscopic experiment and give two examples.
This is needed to disperse the light that has passed through the sample, in order to see which wavelengths of light have been absorbed. A prism or a diffraction grating may be used.
Describe the detector needed in a typical absorption spectroscopic experiment
In the past, photographic plates were used but now highly sensitive cameras are more common. Sometimes the selecting element is rotated and a point detector is used.
Describe the setup of a typical emission spectroscopic experiment
- A sample
- a wavelength selecting element
- detector
The sample is excited and the wavelength selecting element disperses the wavelengths.
The setup is almost the same as that of an absorption spectroscopic experiment, but without the light source.
Describe atomic spectra
The lines show how the electronic energy of atoms is quantised. Different atoms have different spectra, so they can be used to identify elements
Name in order the EM spectrum starting from the lower energy waves
Radiowaves, microwaves, infrared, visable, ultra violet, x-rays, gamma rays
Define an electron volt (eV)
The energy gained by the charge of a single electron moved across an electric P.D of one volt. It is 1.602x10-19 J
Describe how to convert from joules to eV
Divide energy in joules by the value of 1 eV
State the fundamental equation for the speed of an EM wave
c = fλ
c is the speed of the wave f is the frequency λ is the wavelength
State the multiplier for the prefix ‘nano’
10-9
Define one Å
Å represents ‘angstrom’. One angstrom = 1x10-10m
Define wavenumber (v~) and relate it to photon energy
1/λ, measured in (length)-1. We can get E = hcv~, so photon energy is proportional to wavenumber. It is an energy equivalent unit
State how to convert from m-1 to cm-1
Divide by 100
Describe the emission spectra of the hydrogen atom
There are many lines which are clearly in groups (ie series), with each series of lines converging with decreasing wavelengths/ an increasing photon energy. All lines in a series have the same value of n1. The names correspond to the scientists who observed them.