Renal Flashcards
(161 cards)
Renal Functions
Overview
-
Excretion:
- metabolic wastes and compounds excreted in the urine
-
Homeostasis:
- water and electrolyte balance
- extracellular fluid volume
- plasma osmolarity
-
RBC production:
- secretes erythropoietin (EPO)
-
Regulation of vascular resistance:
- Renin secreted by juxtaglomerular apparatus cells
- Initiates RAAS controlling vascular resistance
-
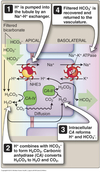
Regulation of acid-base balance
- Secretes or absorbs acids and bases
-
Vit D production
- Calcitriol
-
Gluconeogenesis
- Contributes during prolonged fasting
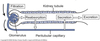
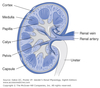
Cortex
Outer part of the kidney which contains:
- Bowmen’s capsules
- glomeruli
- proximal convoluted tubules
- cortical portions of loop of Henle
- distal convoluted tubules
- cortical collecting tubules
- tubules and microvasculature intertwined in a random fashion
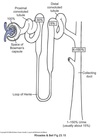
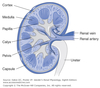
Medulla
Inner part of the kidney composed of pyramids.
Contains:
- medullary portions of loop of Henle
- medullary collecting tubules
- collecting ducts
- tubules and blood vessels arranged in parallel
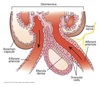
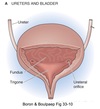
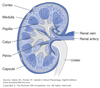
Calyces
- drain into the renal pelvis
- renal pelvis forms the head of the ureter
- ureter sends urine to the urinary bladder
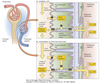
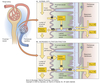
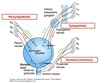
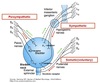
Nephron Structure
Functional unit of the kidney.
-
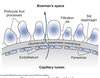
Bowman’s capsule is the start of the nephron
- Blood enters via the afferent arteriole
- Moves into the glomerular capillaries
- Exits via the efferent arteriole
- Filtrate enters Bowman’s space
-
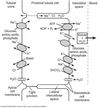
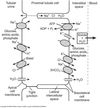
Proximal tubule
- Proximal convoluted tubule (PCT) ⇒ cortex
- Proximal straight tubule (PST) ⇒ medulla
-
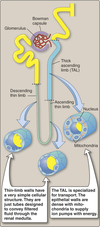
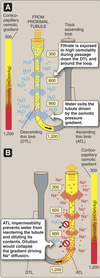
Loop of Henle:
- Descending thin limb (DTL)
- Ascending thin limb (ATL)
- Thick ascending limb (TAL)
- Distal convoluted tubule (DCT)
- Connecting tubule
- Cortical and medullary collecting ducts
- Calyx → renal pelvis → ureter → urinary bladder

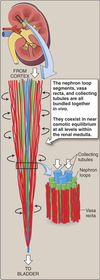
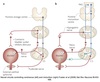
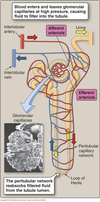
Peritubular Capillaries
- Surrounds the nephron tubule
- Provides O2 and nutrients to tubules.
- Carries away fluid and electrolytes reabsorbed by tubules.
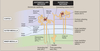
Superificial Nephrons
- Receives ~ 90% of renal blood supply
- Major site of fluid and electrolyte reabsorption
- Short loops of Henle that do not reach the inner medulla
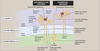
Juxtamedullary Nephrons
- Receive ~ 10% renal blood supply
- Very long loops of Henle that go deep into inner medulla
- Peritubular capillaries forms a long looping vascular network ⇒ vasa recta
- Creates osmotic gradients that concentrate urine
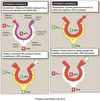
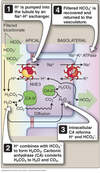
Filtration
Process by which water and solutes leave the glomerular capillaries and enter Bowman’s space.
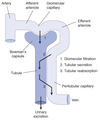
Secretion
Process where substances are transported from the tubular epithelial cells into the nephron lumen.
Substances can be synthesized in the epithelial cells or obtained from surrounding interstitial space.
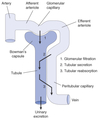
Reabsorption
Process by which substances in the lumen cross the epithelial barrier into the interstitial space where they can be absorbed by peritubular capillaries.
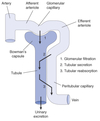
Excretion
The exit of substances from the body via the urine.
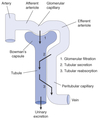
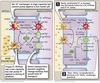
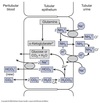
Reabsorption & Secretion
Overview
-
Proximal Convoluted Tubule (PCT)
- Isomolar reabsorption of ~ 70% of filtered water and solute
- Major role in reclaiming salt and water needed to maintain ECF.
- Carrier-mediated Na+ transport.
-
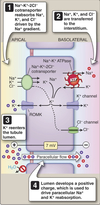
Loops of Henle (LoH)
- Carrier-mediated NaCl reabsorption by the water impermeable thick ascending limb.
- Generation of corticomedullary concentration gradient.
-
Distal Convoluted Tubule (DCT)
- Carrier-mediated reabsorption of NaCl.
- Water impermeable.
- Hormone-modulated Ca++ reabsorption.
-
Connecting Tubule (CNT)
- Both carrier-mediated and ion channel (hormone-regulated) Na+ reabsorption.
- Hormone-regulated Ca++ reabsorption.
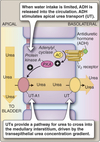
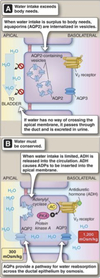
- Arginine vasopressin (AVP)-responsive water permeability.
-
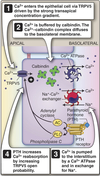
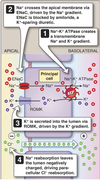
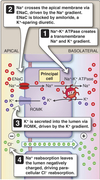
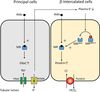
Collecting Tubules and Collecting Ducts
- Different cell types for Na+ reabsorption and H+ secretion.
- Hormone regulated ion channel Na+ reabsorption.
- Hormone modulated K+ secretion.
- Hormone modulated H+ secretion.
- Arginine vasopressin (AVP)-responsive water permeability.
- Urea transport.

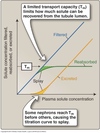
Renal Clearance
The volume of plasma the kidneys completely clear of a substance per unit time.

Glomerular Filtration Rate
(GFR)
The rate at which the kidney’s are filtering.
- Usually corrected to body surface area of 1.73 m2.
- Normal:
- 125 ± 15 ml/min for young adult males
- 110 ± 15 ml/min for young adult females
- GFR declines after age 45-50
- Typically 30-40% lower by age 80
- Used to determine:
- Overall kidney function
- How much a substance is filtered per unit time
- Is a substance reabsorbed or secreted
- What percentage of a filtered substance is reabsorbed
- Assesses overall renal glomerular and net renal tubular function
Inulin Clearance & GFR
Insulin is the gold standard for measuring GFR.
- cleared from the plasma by filtration only
- freely filtered by the glomeruli
- not reabsorbed or secreted by tubular cells
- not created or metabolized by the kidneys
Amount of insulin filtered = amount excreted in the urine.
Clearance of inulin = GFR.
Renal
Mass Balance
-
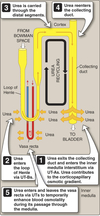
One entrance ⇒ renal artery.
- 20% of plasma filtered into Bowman’s space
- Remainder enters efferent arteriole ⇒ renal vein
-
Two exits ⇒ renal vein and ureter.
- Reabsorbed fluids/solutes ⇒ renal vein
- Substances not reabsorbed enters ureter ⇒ urine
- Substances secreted into tubules ⇒ ureter ⇒ urine
What enters the kidney equals what exits:
Amountfiltered + Amountunfiltered = Amounturine + Amountvein
Amount filtered is GFR x plasma concentration:
Amountfiltered = GFR x Px
Amount excreted is urine flow rate x urine concentration:
Amountx-excreted = ⩒ x Ux
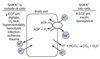
Para-aminohippuric Acid
(PAH)
Used at low concentrations for determination of renal plasma flow rate (RPF) due to these characteristics:
- freely filtered by glomeruli
- any PAH not filtered is transported by tubular cells from peritubular capillaries into urine
- is not synthesized or metabolized by the kidney
* PAH is not naturally-occuring and must be administered by continuous IV.
Renal Plasma Flow Rate
(RPF)
The rate of plama flow through the glomeruli in ml/min.
PAH usually used in RPF determinations.
CPAH = (UPAH x ⩒) / PPAH = RPF
Renal Blood Flow
(RBF)
The amount of blood flow through the kidneys in ml/min.
Calculated from the renal plasma flow rate (RPF) using the hematocrit (Hct) of the blood.
Since Hct is the fraction of blood volume made up of RBC, the remainder is plasma.

Filtration Fraction
(FF)
How much plasma is filtered through the glomeruli compared to how much is not.
Amount of plasma entering the glomerulus = RPF.
Amount of plasma filtered = GFR.
FF = GFR / RPF
Typical filtration fraction ~ 20%.
Applications of Clearance
Comparing clearance of any substance to that of inulin allows determination of overall net action of the renal system on that substance.

Creatinine Clearance
The clinical standard for estimating GFR (eGFR) utilizing empirical equations.
- Creatinine is the muscle creatine phosphate breakdown end-product.
- Daily creatinine production ∝ muscle mass.
- If renal function normal: creatinineexcreted = creatinineproduced so plasmacreatinine fairly constant.
- Very small amount of creatinine is secreted into urine so creatinine clearance slightly overestimates GFR.
- As serum creatinine doubles, GFR decreases by 50%.

Total Body Water
(TBW)
TBW ~ 60% of body weight in lean adult male.
TBW ~ 50% of body weight in lean adult female.
The higher the % body fat, the lower contribution of TBW to body weight.
2/3 of TBW is intracellular fluid (ICF).
1/3 of TBW is extracellular fluid (ECF).
~75% of ECF is interstitial fluid and ~25% is plasma.
Total amount of Na+ in the body determines ECF volume.
Kidneys control Na+ and water reabsorption independently of each other to maintain optimal volume and solute concentrations.