Session 5 Flashcards
(32 cards)
Apply the infection model to a patient presenting with an infection linked to travel
- A 26 year old female presents with rigor (fever, shaking shivery sweats) and headache
- She’d returned 10 days earlier from Malawi where she spent Christmas with her husband
- Within 24 hours, lapsing consciousness
- Taken directly to ED
- Had taken doxycycline prophylaxis for malaria, used insect spray and slept under a tent

To expand the description of pathogen/person/practice/place as it applies to travel-related infections
[*] Key aspects of the travel history
Where did you travel? Whereabouts in the country did you travel?
When?
How (direct/via)? E.g. flight, cruise,
Accommodation? What type? Tent/hotel?
How long was the trip?
Specific risks (including sexual contacts) e.g. eating certain foods in certain areas, swimming in particular rivers
Preventive measures? (Remember they don’t remove the risk but they reduce it)
[*] NB specific answers are needed!

Understand the importance of a travel history
[*] Imported diseases are rare/unknown in the UK
[*] Different strains of pathogen are antigenically different which has implications on protection and detection as some strains may be more antibiotic resistant than others
[*] You also need to consider infection prevention both on the ward (e.g. consider placing patient is isolation) and in the laboratory (hazard for personnel handling patient specimens – they are at risk)
Requirement to consider isolation of patient pending diagnosis
Some conditions do NOT have case-to-case transmission
High-risk specimens
- Potential risk especially laboratory staff
- Requirement to identify high risk samples
- Travel history key element of assessment
[*] Differential Diagnosis from history
- Malaria (endemic to particular areas)
- Typhoid (endemic to particular areas)
- Meningococcal septicaemia (CNS infection – may need a CT scan of head and neck)
- Dengue (large problem in many countries, no specific treatment, potentially serious sequela)
- Non-travel related infection
Describe Malaria and its pathogenesis
[*] 4 main species: Plasmodium falciparum (most serve form of disease), vivax, ovale and malariae
[*] Vector is female Anopheles mosquito
[*] No case-to-case spread but cryptic (people who claimed they have not travelled to endemic areas so mosquito might have been in airway/baggage etc) and iatrogenic (due to hospital care) cases are rarely reported
[*] Occurs in tropics e.g. Africa, Asia, Middle East, South and Central America
[*] 250 million cases and 1 million deaths per year
[*] ~1500 cases per year in UK (in the UK we don’t have the specific mosquitos that carry this parasite so the vast majority of patients would have travelled to an endemic area)
Describe the History, Examination, Investigation and Treatment for malaria
Incubation period 1-3 weeks (or longer – sometimes up to months) after bite (which patient might not recall)
History:
- Headache, cough, fatigue, malaise, arthralgia, myalgia
- Then a more persisting febrile illness: fever chills and sweats which eventually cycle every 3rd (tertian) or 4th (quartan) day (varies for different strains
Examination:
- Other than fever often few signs (+/- splenomegaly)
- Cerebral features – coma
- Respiratory distress (metabolic acidosis, pulmonary oedema).
- There may also be organ disease and failure which could be fatal
[*] Malaria Investigations & Treatment:
- Malaria should be managed by an ID physician
- Blood smear to detect parasites
- FBC, U&Es (to look t renal impairment), LFTs (liver function tests), glucose (which could indicate impairment of the CNS)
- Head CT if CNS symptoms
Treatment depends on species:
- P. falciparum (‘malignant’) – quinine or artemisinin
- P. vivax, ovale, malariae (‘benign’) – choloroquine +/- primaquine (for exo-erythrocytic phase)
- Exo-erythrocytic – outside the blood

Describe the prevention of malaria
Assess risk – knowledge of at risk areas
- Regular/returning travellers (people who were born in these endemic areas and have immigrated)
Bite prevention
- Repellent, adequate clothing, nets
- Chemoprophylaxis before travel
- Must include regular/returning travellers
Chemoprophylaxis:
- Specific to region
- Start before (at least a week or two) and continue after return (generally for at least 4 weeks)
Describe Enteric Fever (Typhoid and paratyphoid)
[*] Widespread distribution particularly in the tropics – poor sanitation
[*] Mainly Asia, Africa and South America
[*] 21 million cases per year – mainly children
[*] UK travel-related (rarely transmission)
~500 cases/year (mainly Indian subcontinent)
[*] Mechanism of infection: faecal-oral from contaminated food/water (where there isn’t sufficient segregation between drinking water supply and sewage system), sources is cases or carriers (human pathogen only)
What causes Enteric Fever?
[*] Salmonella – the organisms
- Salmonella enterica serovar
- Typhi/Paratyphi A, B or C
- Enterbacteriaceae, Aerobic Gram-negative rod
- Closely related to E. coli
- Non-lactose fermenter
Virulence
- Gram-negative endotoxin (released when organism breaks down), VI antigen
- Invasin – protein which allows intracellular growth
- Fimbriae adhere to epithelium over ileal lymphoid tissue (Peyer’s patches) => reticuloendothelial system
What are the Signs and Symptoms, Investigations and Treatment for Enteric fever?
[*] Enteric fever – symptoms and signs
- Systemic disease with fever and headache
- Incubation period 7-14 days
- Abdominal discomfort, constipation, dry cough
- Hepatosplenomegaly (enlarged liver and spleen), occasionally rash
- Relative bradycardia (normally with a fever, a patient has a raised pulse rate)
- Complications include intestinal haemorrhage and perforation around Peyer’s patches
- Paratyphoid generally milder
[*] Enteric fever – Investigations
- Moderate anaemia
- Relative lymphopenia
- Raised Liver Function Tests (transaminase and bilirubin)
- Culture from blood and faeces
- Serology (antibody detection) is no longer used due to so many cross reactions
[*] Enteric fever – Treatment
- Originally chloramephenicol, then ampicillin or co-trimoxazole
- Resistance led to use of ciprofloxacin (a quinolone drug)
- Ciprofloxacin resistance is now common
- Usually treated with ceftriaxone or azithromycin (a macrolide) 7-14 days (IV treatment)
Describe the prevention of Enteric Fever
- Food and water hygiene precautions (segregation of drinking water and sewage control)
- Typhoid vaccine for high-risk travel, laboratory personnel
- Vi capsular polysaccharide antigen OR live attenuated vaccine (strain produces an immunogenic reaction devoid of pathogen)
- Modest protective effect (50-75%)
Describe non-typhoidal salmonella infections
- ‘Food-poisoning’ salmonellas
- Widespread distribution including UK
- Different strains e.g. Salmonella typhimurium, Salmonella enteritidis
- Primarily GI disease presentation: diarrhoea, fever, vomiting, abdominal pain
- Generally self-limiting but bacteraemia and deep-seated infections may occur (particularly in immunocompromised patients)
Describe Brucellois
[*] Brucellosis: a zoonosis
- Primary animal pathogen
- Brucella abortus (cattle), B. melitensis (goats and sheep) Gram-negative coccobacillus
- South Europe, Africa, Asia, Central and South America
- Transmission through skin breaks / GI tract (milk)
- Non-specific febrile illness (‘undulant fever’)
- Bone/joint involvement, epidydimitis (swollen painful testes)
- Diagnosis generally from blood culture
- Treatment with doxycycline and rifampicin
Describe emerging novel viruses and Ebola
[*] Emerging Diseases – Novel viruses
Influenza pandemics:
- 1918-19 ‘Spanish’ 1957 ‘Asian’ 1968 ‘Hong Kong’
- H5N1 (‘avian flu’) in 2005
- H1N1 (‘swine flu’) in 2009
- H7N9 in 2013 – another avian strain
- Many of these newer influenza strains have been associated with particular parts of the world so TRAVEL HISTORY is very important
Novel coronaviruses
- SARS-CoV in 2003 Severe Acute Respiratory Syndrome (patient often ended u on a ventilator in ITU)
- MERS-CoV in 2012 Middle East Respiratory Syndrome (need to be isolated to reduce the risk of droplet transmission)
[*] Viral Haemorrhagic Fever – Ebola:
- First described in Congo in 1976
- Filovirus related Marburg haemorrhagic fever
- Flu-like illness wit vomiting, diarrhoea, headaches, confusion, rash
- Internal; external bleeding at 5-7 days
- Spread by direct contact with body fluids (but only from patients who are symptomatic)
- Ebola 2014: index case Dec 2013
- Initial source possible bats
- Thought to have spread from Central Africa => now moved across to West Africa (Sierra Leone, Liberia and Guinea)
- Treatment experimental (Zmapp – monoclonal antibodies and antivirals)
- Vaccine under development

What non-infection causes of Travellers’ Diarrhoea may there be? And what kinds of diarrhoea are there?
It is also worth remember non-infectious causes of diarrhoea that can occur in travellers:
- Inflammatory Bowel disease
- Irritable Bowel syndrome
- Diverticular disease
- Malignancy
- Drug related (particularly antibiotic related)

To understand where and how to look up information on travel-related infections
[*] http://www.hpa.org.uk/ (Public Health England)
[*] http://www.cdc.gov/outbreaks/ (Centre for Disease Control - US)
[*] www.who.int/ (World Health Organisation)
[*] www.nathnac.org/pro/ (National Travel Health Network. Very useful resource for all aspects of travel medicine)
Describe influenza virus and its transmission
[*] Influenza viruses are RNA viruses that make up three of 5 genera of the family orthomyxoviruses
- Influenza A are the most virulent human pathogens and cause the most severe disease. It infects humans, other mammals and birds, and causes all flu epidemics. It is a spherical, enveloped RNA virus
- Influenza B infects humans and seals
- Influenza C infects humans, pigs and dogs
[*] Most common symptoms are chills, fever, runny nose, sore throat, muscle pains, headache, coughing, weakness/fatigue and general discomfort
[*] Can be transmitted among humans in three ways
- Direct contact with infected individuals
- Contact with contaminated objects (called fomites, such as toys, doorknobs) – respiratory droplets
- Inhalation of virus-laden aerosols
Describe Influenza A. What is meant by antigenic shift and antigenic drift?
[*] Influenza A can subtyped depending on the antigen associated with its outer viral proteins (Haemagglutinin (H) and Neuraminidase (N)). So far 16H and 9N antigen subtypes have been identified. Subtypes of the influenza A virus are designated by the unique combinations of H&N antigens they process e.g. H1N1
[*] The antigens on the influenza viruses can vary via processes of antigenic drift and antigenic shift. This creates new subtypes of the virus which can sometimes be extremely virulent and often deadly.
- Antigenic Drift: minor changes that occur in H & N proteins caused by random mutations in the viral RNA. Does not change the subtype
- Antigenic Shift: this is the process where by one cell is infected with two different subtypes of the influenza virus. Reassortment of the viral RNA from both subtypes leads to the creation of new subtypes of the virus. Estimated to happen roughly every 10-20 years.
Describe Legionella pneumophilia and an example of its clinical important
[*] Gram negative rods
[*] Facultative intracellular parasite
[*] Aerobic
[*] Normally found within environmental protozoa and amebae within soil and water systems
[*] Infection is normally spread via aerosol spread – inhalation. Human to human transmission does not occur so no requirement to isolate patient
[*] The bacteria are phagocytised by macrophages but prevent the phagosome from fusing with a lysosome so evades destruction by the macrophage and instead uses the macrophage as a site to replicate and spread.
[*] A clinical example is Legionnaires’ disease (pneumonia) caused by Legionella pneumophila serogroup 1 (an atypical pathogen)
[*] Amend empiric treatment to specific treatment – usually a quinolone class antibiotic e.g. levofloxacin
[*] Legionnaire’s disease is a notifiable disease
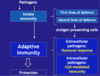
Describe the key features of Antigen-Presenting Cells (key players in the immune response)
Strategic location (B and T cell interaction)
Skin (SALT)
- Mucous membranes (GALT, NALT, BALT)
- Lymphoid organs (Lymph nodes, spleen)
- Blood circulation (plasmacytoid and myeloid DCs)
Pathogen capture:
- Phagocytosis (whole microbe) via opsonisation and complement - specific
- Micropinocytosis (soluble particles) – invagination of the membrane surrounding soluble particles - random non specific
Diversity in pathogen sensors (PRRs) - recognition
- Extracellular pathogens (bacteria)
- Intracellular pathogens (viruses)
Different Types of APCs:

What do APCs do?
Capture/Processing/Presentation (to T cells to initiate the most appropriate immune response)
- Extracellular microbes have specific PAMPs which are processed by Major Histocompatibility Complexes (MHCs) on the membranes of APCs => leads to activation of Humoral Immunity (antibodies, complement)
- Intracellular Microbes have specific PAMPs which are processed by MHCs on APCs => leads to activation of Cell-Dependent Immunity (Cytotoxic T/T Killer cells macrophages, antibodies)

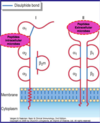
Where are MHCs found?
Major Histocompatibility Complex (MHC) or Human Leukocyte Antigen (HLA)
Class I molecules: found on all nucleated cells
Class II molecules: found on dendritic cells, macrophages, B cells

What are key features, structure and functions of MHC Class II molecules?
- Co-dominant expression: both parental genes are expressed which leads to an increase in the number of different MHC molecules (maximises the number and array of different MHC molecules to recognize different microbes)
- Polymorphic genes: different alleles among different individuals lead to an increased presentation of different antigens/microbes – we each have around 10 trillion different MHCs
Main Function:
- MHC Class I: present peptides from intracellular microbes (viruses)
- MHC Class II: present peptides from extracellular microbes (bacteria)
Structure of MHC Class I and Class II molecules
- Peptide binding cleft: variable region with highly polymorphic residues
- Broad specificity: many peptides presented by the same MHC molecule (can present different peptides from the same microbe as well as different microbes)
- Responsive T Cells: MHC Class I present to CD8+ T cells and MHC Class II present to CD4+ T Cells
What are the antigen-processing pathways and why are some patients special?
- Endogenous Pathways (all cells): if there is a match between MHC Class I and peptide this will lead to a complex formed which will express viral and cell proteins on surface. One of the T cells senses proteins and signals activation of CD8 cells. The microbes are killed.
- Exogenous pathways: never present in the cytosol – only present in endocytic vesicles. Lysozome chops up endosome full of microbial peptide which is recognized by T cells, activating CD4+ cell
- They are better equipped to next infection because the cells have the right specific MHC molecule => able to initiate right response
- Some patients are called elite controllers or long term nonprogressors (LTNP) and can control viral replication – able to main high, normal CD4+ T count with a controlled viral load.

Explain about HLA Typing and the anti-HIV Responses
MHCs are also known as Human Leukocyte Antigens (HLA). HLA typing and anti-HIV responses:
- Slow progressors present key peptides for the survival of the virus that the virus can’t mutate so you are able to mount an immune response (body is able to recognize the virus affected cells)
- Rapid progressors present less critical peptides so mutation occurs => weak T cell response