Stereochemistry Flashcards
(72 cards)
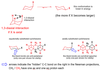
Reaction of an aldehyde and a cyanide…
How many three dimensional products can be created? Why?
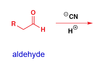
Two: CN- (nucleophile) can attack (electrophilic - electrons pulled by O of carbonyl) carbon from above or below the plane of the carboynyl group. This will change the orientation of bonds relative to each other as seen in the image.
What is the definition of an enantiomer?
Stereoisomers that are not identical but are mirror images of each other, and which are chiral (their mirror images cannot be superimposed on each other - like left and right hands palm-down!)
Achiral reactants that create chiral products form these enantiomers as a _______ mixture. What does this mean?
Racemic
50/50 of each enantiomer
How can you tell a chiral from an achiral structure?
Look for planes (NOT LINES) of symmetry - any structure with such a plane can’t exist as two enantiomers, and is achiral.
Most biological receptors are chiral or achiral? Why?
Chiral - because they are made up from chiral building blocks such as amino acids.
Define: constitutional isomers and stereoisomers
C: the way atoms are connected up differs
S: the atoms have the same connectivity, but are arranged differently (eg: enantiomers or E/Z [double bond] isomers)
What is the difference between configuration and conformation?
Are enantiomers a different configuration or a different conformation?
Changing the configuration of a molecule always means that bonds are broken (different config = different molecule).
Changing the conformation of a molecule means rotating about bonds (different conforms = interconvertible = same molecule)
Enantiomers = different configurations (as you have to break bonds to turn one enantiomer into the other)
What is a stereogenic/chiral centre? Will it have any planes of symmetry?
A carbon (usually) atom carrying four different groups
No (that’s why it can be called a chiral centre)
What do wiggly bonds indicate?
That is referring to both stereoisomers (a racemic mixture of enantiomers), or unknown stereochemistry
What is Strecker synthesis?
How does this differ to natural synthesis of its products?
Process in lab to create an amino acid (it will be a racemic mixture)
In nature, amino acids are usually just one of the enantiomers (L-amino acids … or mostly S, except for cysteine)
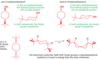
What are the Cahn-Ingold-Prelog rules used for?
How do they work?
Figuring out whether we are looking at an R or S enantiomer
1) Number substituents of sterogenic centre according to priority (higher atomic numbers = rank 1)
2) Rotate so that lowest priority points away from you
3) Draw a circle from priority 1 to priority 2 to priority 3. If the circle is moving rightwards/clockwise, it is R. If it is leftwards/anti-clockwise, it is S.
Cahn-Ingold-Prelog rules for R and S enantiomers: what is the fast way of doing it (once you have calculated priorities)?
Switch the lowest priority substituent with whichever substituent is facing away from you (dashed line), draw the little circle thing, and just take the OPPOSITE result (so, if it looks like R, the answer is S, and vice versa)
[NB: may not work, so just try and rotate]
Cahn-Ingold-Prelog rules for R and S enantiomers: what two ways are there of handling double/triple bonds that are attached to the substituent atoms (when calculating priority)?
Simple: just counts as another version of a single bond (so [C]=C and C-[C]-C would both be CCH)
Complex: involves ‘ghost’ atoms (A=B is considered to be singly bound to B and and a copy of B [the ghost] that isn’t bound to anything else), but it won’t be needed for this course… plus, you don’t wholly get it anyway.
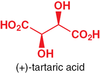
Enantiomers have identical properties in regards to what? What important difference is there?
Identical: physical properties (eg: melting points) and spectroscopical properties (UV, IR, NMR, MS)
Different: they rotate plane-polarized light in opposite directions
Can enantiomers be separated by traditional chromatographic techniques (TLC, GC, HPLC)?
No.
Normal light has electric and magnetic field components in ___ planes. How do you get plane-polarized light? What do you use to measure it? What is the name for this number (that is specific to each compound)? What does the sign (+/-) indicate?
All
By putting it through a specific filter. You measure the specific rotation by using a polarimeter (+ = right, - = left)
How do you calculate specific rotation?
[Alpha] = rotation / concentration (g cm-3)*path length (dm)
1 dm = 10 cm
28mg dissolved in 1mL of ethanol and transferred into a 10cm long polarimeter cell. Observed rotation is -4.35 degrees. What is the specific rotation?
[alpha] = alpha/concentration*path length
1mL = 1 cm-3 ——> 28mg/cm-3 -> 0.028g/cm-3
10cm = 1 dm
-4.35/1*0.028 = -155.4
(S)-(+)-alanine
Is there any correlation between +/- and R/S? Explain.
No. +/- is an inherent physical property of each optically active compound, whereas R/S is based on an artificial nomenclature system (that refers to its absolute configuration)
What is the difference between absolute and relative configuration?
Absolute configuration represents the precise arrangement of substituents at a stereogenic center (as calculated via R/S, or using techniques such as x-ray crystal structure, spectroscopy, etc.
Relative configuration represents the arrangement of something relative to something else (eg: cis/trans definition is talking about the position of two groups/atoms relative to eachother, or the old way they used to calculate D and L)
(S)-(+)-alanine
What would its enantiomer be?
(R)-(-)-alanine
There may be no correlation between R/S and +/-, but the enantiomers of a given compound will always be opposite…
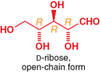
Is it possible to assign absolute stereochemistry from +/- or D/L?
Is it possible to assign +/- or D/L from a structure?
Are D/L and +/- connected?
No and no and no. D/L is only always the same as +/- for glycerinaldehyde (which used to be used–via relative stereochemistry–to assign it to other compounds… it was not always effective, which is why D/L aren’t used anymore…)
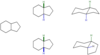
What are diastereoisomers?
How are they different to enantiomers?
Stereoisomers that are not enantiomers (they are neither mirror images nor super-imposable)
They have different physical and spectroscopic properties (unlike enantiomers, which only differ in how they twist plane-polarized light)

What does it mean for a molecule to be cis/trans?
How does the E/Z system differ?
In nomenclature, “cis” is used to distinguish the isomer where two identical groups (e.g. the two chlorines in 1,2-dichlorocyclopentane) are pointing in the same direction from the plane of the ring/double bond, and trans to distinguish the isomer where they point in opposite directions.
E/Z system assigns priorties (using Cahn-Ingold-Prelog) to the substituents of the atoms connected on either side of the double bond. ‘E’ (= Enemies) refers to the higher priority groups being on opposite sides of the double bond, and ‘Z’ refers to them being on the same side.