Struggle Deck Flashcards
(38 cards)
Will a halogen make a stronger bond with a more or less substituted carbon?
less substituted
allyl group

True/False: Some optically active compounds are not polar.
False
In deuterated cyclohexane, is D more stable axial or equatorial?
axial
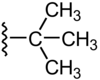
Isopropyl group
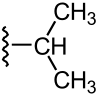
How does increased branching in a hydrocarbon affect its density?
Density increases.
IR absorbance for carboxylic acid
broad peak around 2800 cm-1
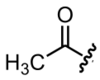
What is an oxo substituent?
It is a ketone group.
ethoxy group

What defines a primary amine?
a nitrogen bonded to 2 hydrogens and one carbon
How does an electron-withdrawing group affect acidity and basicity?
It increases acidity and decreases basicity.
An ester is treated with acidic water. What are the products?
It undergoes hydrolysis to produce an alcohol and a carboxylic acid.
vinyl group

How, specifically, does increasing temperature affect reaction rates?
It increases reaction rates by providing greater energy for breaking bonds.
What does a higher numerical value of heat of hydrogenation (delta H) imply about the stability of an alkene?
it is less stable
Which is the strongest acid: H on an sp-hybridized or sp3-hybridized atom?
H on an sp-hybridized atom
benzyl group

Is a more or less substituted alkene stronger?
more substituted
What does an equilibrium constant indicate when it is greater than 1?
The reaction is favorable in the forward direction (there are more products than reactants).
Which is the strongest base: a lone pair on an sp-hybridized atom or sp3-hybridized atom?
lone pair on an sp3-hybridized atom
Does a more or less stable reactant produce a higher heat energy upon reaction?
less stable
What does CrO3 with an acid do to a compound?
It can oxidize the compound.
Which is the strongest nucleophile? - ester - ether - amide - amine
amine
What is the unbalanced equation for the combustion of a hydrocarbon?
CH4 + O2 ► CO2 + H2O