Test on States of Matter Flashcards
(39 cards)
What is the shape of an atom?
Spherical
What does an atom consist of ?
Draw it?
Three types of particles(sub-atomic particles)
proton, neutrons, electrons
Which sub-atomic particle is positively charged?
Protons
Which sub-atomic particle has no charge?
Neutrons
Which sub-atomic particle is negatively charged?
Electrons
Explain 3 pieces of evidence that matter is made up of tiny particles.
- Brownian Motion
- Diffusion
- Osmosis
What is Diffusion?
Diffusion is the movement of particles from an area of high concentration to an area of low concentration until the particles are evenly distributed.
This process occurs in both GASES and LIQUIDS

What is Osmosis?
Osmosis is the movement of water particles from an area of high water concentration to an area of low water concentration, through a semi-permeable membrane.
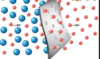
Describe an experiment to explain Osmosis.
You should notice that the level of liquid in the thistle funnel rose.
This occurred because the water particles moved from an area of high concentration inside the funnel.
The semi-permeable membrane has very tiny pores. These are big enough for water particles to pass through but too small for the larger sugar particles to pass through.

Describe an experiment to show diffusion.
In the experiment, there was a high concentration of food coloring particles in the drop of food coloring.
The particles diffused from this area of high concentration to distribute themselves evenly in the beaker of water.

Describe an experiment with Diffusion in GASES.
Diffusion can also be observed in gases.
When a jar filled with brown bromine vapour is allowed to mix with another jar containing air particles, bromine vapour spreads into the two jars. (Figure 1.2.)
Particles of bromine move from a high concentration to a low concentration until they are evenly distributed.

Describe an experiment showing the Brownian Motion.

In 1827, the botanist Robert Brown using a high-powered microscope observed the CONSTANT IRREGULAR MOTION OF POLLEN FLOATING ON WATER.
He called this Brownian Motion.
This motion can be explained in terms of kinetic theory.
Air and water particles are constantly moving.

Three factors that affect the rate of diffusion?
- If the water or substance
The particulate model of matter states?
That matter is made up of small discrete particles and that the discrete particles in matter are in constant and random motion.

B

liquid to gas

Condensation

B

B

C

C

D

Evaporation and diffusion

D

















