Tuesday [abdomen cavity, liver physiology, hernias] Flashcards
(95 cards)
Where is Calot’s triangle located?
Porta hepatis of the liver [where the hepatic ducts and neurovascular structures enter/exit the liver]
What are the border’s of Calot’s trinalge?
- medial = common hepatic duct - inferior = cystic duct - superior = inferior surface of the liver
Annotate Calot’s borders


Contents of Calot’s triangle
- Right hepatic artery – formed by the bifurcation of the proper hepatic artery into right and left branches. - Cystic artery – typically arises from the right hepatic artery and traverses the triangle to supply the gall bladder. - Lymph node of Lund – the first lymph node of the gallbladder. - Lymphatics
What is a laraproscopic cholecystecomy procedure and why is Calot’s trinalge relevant to this?
The triangle of Calot is of clinical importance during laparoscopic cholecystectomy (removal of the gall bladder). In this procedure, the triangle is carefully dissected by the surgeon, and its contents and borders identified. This allows the surgeon to take into account any anatomical variation and permits safe ligation and division of the cystic duct and cystic artery. Of particular importance is the right hepatic artery – this must be identified by the surgeon prior to ligation of the cystic artery. If Calot’s triangle cannot be delineated (such as in cases of severe inflammation), the surgeon may elect to perform a subtotal cholecystectomy, or convert to open surgery.
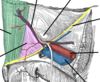
Borders of the inguinal triangle
The inguinal triangle is located within the inferomedial aspect of the abdominal wall. It has the following boundaries: Medial – lateral border of the rectus abdominis muscle. Lateral – inferior epigastric vessels. Inferior – inguinal ligament
Contents of the inguinal triangle
Other than the layers of the abdominal wall, the inguinal triangle does not contain any structures of clinical importance.
What does the inguinal triangle demarcate?
However, the triangle does demarcate an area of potential weakness in the abdominal wall – through which herniation of the abdominal contents can occur
Annotate contents of the inguinal triangle
image

Define a hernia
A hernia is defined as the protrusion of an organ or fascia through the wall of a cavity that normally contains it. The inguinal triangle represents an area of potential weakness in the abdominal wall, through which herniation can occur.
What is a direct inguinal hernia?
In a direct inguinal hernia, bowel herniates through a weakness in the inguinal triangle, and enters the inguinal canal. Bowel can then exit the canal via the superficial inguinal ring and form a ‘lump’ in the scrotum or labia majora. Direct hernias are acquired (usually in adulthood), due to weakening in the abdominal musculature
What is an indirect inguinal hernia?
This is in contrast to an indirect inguinal hernia – where bowel enters the inguinal canal via the deep inguinal ring. By definition, a direct inguinal hernia occurs medially to the inferior epigastric vessels (through the inguinal triangle), and an indirect hernia occurs laterally to these vessels
Define these types of hernias


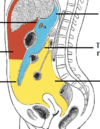
What is the peritoneal cavity?
Potential space between teh parietal and visceral peritoneum.
What does the peritoneal cavity contain?
It normally contains only a thin film of peritoneal fluid, which consists of water, electrolytes, leukocytes and antibodies. This fluid acts as a lubricant, enabling free movement of the abdominal viscera, and the antibodies in the fluid fight infection. While the peritoneal cavity is ordinarily filled with only a thin film of fluid, it is referred to as a potential space because excess fluid can accumulate in it, resulting in the clinical condition of ascites (see clinical applications)
Peritoneal cavity

image

Greater sac divided by what?
Transverse colon
How is the greater sac subdivided?
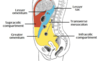
Greater Sac The greater sac is the larger portion of the peritoneal cavity. It is further divided into two compartments by the mesentery of the transverse colon (known as the transverse mesocolon): Supracolic compartment – lies above the transverse mesocolon and contains the stomach, liver and spleen. Infracolic compartment – lies below the transverse mesocolon and contains the small intestine, ascending and descending colon. The infracolic compartment is further divided into left and right infracolic spaces by the mesentery of the small intestine
How are the supra and infra-colic compartments connected?
The supracolic and infracolic compartments are connected by the paracolic gutters which lie between the posterolateral abdominal wall and the lateral aspect of the ascending or descending colon.
Greater sac


What are subphrenic abscesses?
The subphrenic recesses are potential spaces in the supracolic compartment of the greater sac. They are located between the diaphragm and the liver. There are left and right subphrenic spaces, separated by the falciform ligament of the liver.
Where do subphrenic abscesses typically occur?
Subphrenic abscesses refer to an accumulation of pus in the left or right subphrenic space. They are more common on the right side due to the increased frequency of appendicitis and ruptured duodenal ulcers (pus from the appendix can track up to the subphrenic space via the right paracolic gutter).
Where does the lesser sac [omental bursa] lie?
The lesser sac lies posterior to the stomach and lesser omentum. It allows the stomach to move freely against the structures posterior and inferior to it
How is the omental bursa connected to the greater sac?
The omental bursa is connected with the greater sac through an opening in the omental bursa – the epiploic foramen (of Winslow)