Week 1- Carb Metabolism Flashcards
(56 cards)
What does IAPP do? What does PP do?
Islet amyloid polypeptide- secreted by beta cells- retards gastric emptying, inhibits glucagon secretion. Pancreatic polypeptide (secreted by PP cells) reduces appetite and food intake.
What is the relative composition of islets (how much of each cell type present), and what is the distribution
60% beta cells (insulin +IAPP) 30% alpha cells (glucagon) <5% PP cells (pancreatic polypeptide) Cells are scattered, but closely associated with microcirculation
What is the innervation of the islets and what does it promote?
PNS (vagus nerve (ACh)→ promote insulin and glucagon release)
SNS (post-ganglionic celiac (NE)→ inhibit insulin release, promote glucagon)
What % of liver is glycogen when the liver is saturated?
~5%, after this glucose is shunted to FA synthesis and is stored in adipocytes as triglycerides
What kind of reeptor is the insulin receptor?
tyrosine kinase
What are the hormones involved in normal glucose counter regulation?
Glucagon and epi are fast acting, GH and cortisol are slow acting

Etiologies of Diabetes Mellitus

Is beta cell mass dynamic or static througout life? When does diabetes occur?
Beta- cell mass is dynamic throughout life (e.g. increases in pregnancy, obesity and insulin resistance)
Diabetes occurs at 20-30% of normal (“critical beta cell mass”)

What is the pathogenesis of T1DM?
Autoimmune destruction of beta cells- during the initial attack they upregulate division and divide faster than a non-diabetic pancreas
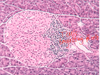
What is the pathogenesis of T2DM?
- amyloid deposition (plaques of IAPP)
- macrophage activation –> inflammation (vs. T-cell mediated in T1DM)
peripheral insulin resistance, maybe related to inflammation in adipose tissue
beta-cell exhaustion
glucotoxicity

What happens to islets in chronic pancreatitis?
Eventually they fibrose and become isolated from blood supply

How does cystic fibrosis cause pancreatic fibrosis?
Loss of CFTR acidifies pancreatic secretions and proteins precipitate causing ductal obstruction–> fibrosis. This can lead to DM by bystander damage
How much does DM shorten life expectancy by, on average?
15 years!
How does the clinical presentation differ between T1DM and T2DM?
T1DM
- usually younger, lean, acute, ketosis prone, may not have FHx
T2DM
- usually older, progression presentation, overweight, not ketosis prone with a FHx of DM
Possible presenting symptoms of DM
Sx of DM
• thirst
• polyuria
• recent weight gain or loss
• extreme fatigue
• blurred vision
• recurring infections
• cuts/bruises slow to heal
• tingling or numbness in hands or feet
• ED in males
Diagnostic criteria for DM
Diagnostic criteria
• Any of the following with symptomatic hyperglycemia:
o FPG > 7
o A1C > 6.5.%
o 2hPG in OGTT >11.1
o a random PG of >11.1
• Any of the above, without Sx, repeated on another day
What other autoimmune conditions must be monitored for in someone with T1DM?
- Hashimoto’s thyroiditis (hypo)
- Grave’s (hyper)
- Addison’s (adrenal hypofunction)
- Coeliac
Why is it important to try and classify T1DM vs. T2DM if you are unsure?
T1DM will benefit from early induction of insulin (don’t need to try oral hypoglycemics)
What are conditions associated with DM?
- obstructive sleep apnea
- polycystic ovarian syndrome
- acnthosis nigricans
- HIV infection
- psych disorders
Screening guidelines for DM
Screening guidelines:
• Everyone over 40, every 3 yrs if normal results
• Younger for
o High risk ethnic group (FN, Hispanic, Asian, African descent)
o 1st deg relative with DM
o previous gestational
o CV risk factors
o Drugs (atypical antipsychotics, ARVs, glucocorticoids)
o Associated diseases (PCOS, acantosis nigricans, OSA, psych, HIV)
Precipitants of diabetic ketoacidosis and hyperosmolar hyperglycemic state
The I’s
- Insulin deficient (failure to take enough)
- Iatrogenesis (e.g. glucocorticoids)
- Infection (this increases epi, cortisol)
- Inflammation (e.g. pancreatitis, cholecystitis)
- Ischemia/Infarction (MI, gut, cerebral)
- Intoxication (drugs, EtOH)
What would you expect on the following lab tests for a pt with DKA?
- lytes (Na, K, HCO3
- pH
- Urea
- Creatinine
- Glucose
- pCO2
lytes
- pseudohyponatremia: water pulled osmotically from intracellular compartment dilutes the sodium
- normal to hyperkalemia, with depletion of total body potassium because acidosis pulls K out of cells and it is subsequently lost in osmotic diuresis
- HCO3- is low because of the ketoacidosis
pH
- acidic because of the ketoacidosis
Urea
- high…don’t know why
Creatinine
- high…volume reduction decreases GFR
Glucose
- high because a relative or absolute lack of insulin promotes gluconeogenesis, glycogenolysis and prevents uptake into cells –> hyperglycemia
pCO2
- low, because you’re blowing off CO2 in an attempt to correct the metabolic acidosis
What are some differentiating factors between DKA and HHS?
• Differentiating factors:
o No ketoacidosis in HHS, negative ketones
o HHS tends to have higher blood glucose
o HHS tends to have more profound volume contraction → higher POsm
And, DKA is T1DM while HHS is more elderly T2DM
Management of DKA and HHS
o Management
• Restore volume with isotonic saline
• Bicarb if pH < 7.0 (seen in DKA only)
• Bolus–> continuous infusion insulin
• Replace potassium
• Go slow and monitor POsm, Na, K, HCO3, PO4, glucose !










