Block 2 presentation 1 - Cell Bio Transduction part 1 Flashcards
(80 cards)
Signal transduction:
Signal transduction is Detection of specific signals at the cell surface and the mechanism by which such signals are transmitted into the cell’s interior, resulting in changes in cell behavior and/or gene expression
The different type of chemical signals that can be received by a cell
Different types of chemical signals can be received by cells
Ligand: substance that binds to a specific receptor, thereby initiating the particular event or series of events for which that receptor is responsible
Primary messenger: A molecule that binds to a receptor, thereby beginning the process of transmitting a signal to the cell. they have a short half life.
Second messenger: any of several substances, including cyclic AMP, calcium ions, inositol triphosphate, and diacylglycerol, that transmit signals from extracellular signaling ligands to the cell interior
Types of Chemical Messengers
Types of Chemical Messengers
Amino acids or their derivatives, Peptides, Proteins, Fatty acids, Nucleosides or nucleotides, Steroid hormones, Retinoids, eicosanoids
Extracellular Signal Molecules Can Act Over what distances? explain.
Extracellular Signal Molecules Can Act Over Either Short or Long Distances
Endocrines:
Each endocrine hormone is secreted by a specific cell type, enters the blood, and exerts its actions on specific target cells, which may be some distance away.
Paracrines:
- Paracrine actions are those performed on nearby cells, and the location of the cells plays a role in the specificity of the response
- Very important in limiting immune response to a specific location in the body
Autocrines:
Autocrine actions involve a messenger that acts on the cell from which it is secreted, or on nearby cells that are the same type as the secreting cells.
Endocrine Signaling
Endocrine cells secrete hormones into the blood, and these act only on those target cells that carry the appropriate receptors: the receptors bind the specific hormone, which the target cells thereby ”pull” from the extracellular fluid
At what speed do extracellular signals move?
Extracellular signals can act slowly or rapidly to change the behavior of a target cell
- Certain types of signaled responses, such as increased cell growth and division, involve changes in gene expression and the synthesis of new proteins; they therefore occur slowly, often starting after an hour or more
- Other responses-such as changes in cell movement, secretion, or metabolism – need not involve changes in gene transcription and therefore occur much more quickly, often starting in seconds or minutes; they may involve the rapid phosphorylation of effector proteins in the cytoplasm
Signal transduction pathways can amplify the cellular response to?
Signal transduction pathways can amplify the cellular response to an external signal
Different types of cells usually respond differently to the same extracellular signal molecule
A cell’s response to extracellular signals depend not only on the receptor proteins it possesses but also on intracellular machinery by which it integrates and interprets the signal it receives
Additional information (not required)
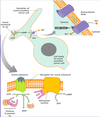
Example neurotransmitter acetycholine:
Different cell types are specialized to respond to acetylcholine in different ways.
- In some cases (B & C), the receptors for acetylcholine differ.
- In other cases (B & D), acetylcholine binds to similar receptor proteins, but the intracellular signals produced are interpreted differently in cells specialized for different functions.
The fate of some developing cells depends on?
The fate of some developing cells depend on their position in morphogen gradients
additional info (not required)
The same molecule acting on the same cell type can have qualitatively different effects depending on the signal’s concentration. This phenomenon is extremely important during development
A morphogen is an extracellular signal molecule which diffuses out from a localized cellular source, generating a signal concentration gradient to which the cells response will be dependent upon.
Example shown here: The different concentrations of morphogen induces expression of different sets of genes, resulting in different cell fates.

Each cell is programmed to respond to?
Each cell is programmed to respond to specific combination of extracellular signal molecules
additional info (not required)
Each cell displays a set of receptors that enables it to respond to a corresponding set of signal molecules produced by other cells.
These signal molecules work in combinations to regulate the behavior of the cell.
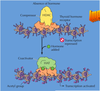
As shown here,
- an individual cell often requires multiple signals to survive (blue arrows)
- and additional signals to grow and divide (red arrows) or
- differentiate (green arrows).
If deprived of appropriate survival signals, a cell will undergo a form of cell suicide known as apoptosis.

Mechanisms in which target cells can become desensitized to an extracellular signal molecule
Mechanisms in which target cells can become desensitized to an extracellular signal molecule include:
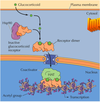
- By hiding away the receptor until it’s needed again ( in the recycling endosome). ex insulin signaling
- By destroying the receptor (get sorted in the early endosome and get sent to the late endosome and goes to the lysosome to get degraded.
- shutting off the pathway by various ways like negative feedback loop
- Cell can adjust their sensitivity to a signal
- In responding to many types of stimuli, cells and organisms are able to detect the same percentage of change in a signal over a wide range of stimulus strengths.
- This is accomplished through a reversible process of adaptation or desensitization, whereby a prolonged exposure to a stimulus decreases the cells’ response to that level of stimulus
Explain The binding of extracellular signal molecules to either cell-surface or intracellular receptors
The binding of extracellular signal molecules to either cell-surface or intracellular receptors
Most signal molecules are hydrophilic and are therefore unable to cross the target cell’s plasma membrane directly; instead, they bind to cell-surface receptors, which in turn generate signal inside the target cell
Some small signal molecules, by contrast, diffuse across the plasma membrane and bind to receptor proteins inside the target cell- either in the cytosol or in the nucleus.
- Many of these small signal molecules are hydrophobic and nearly insoluble in aqueous solutions; they are therefore transported in the bloodstream and other extracellular fluids bound to carrier proteins, from which they dissociate before entering the target cell.
Steroid Receptor Signaling
Steroid Receptor Signaling
There are now two known signaling mechanisms for steroid hormones
1) Nuclear-initiated steroid signaling (Classical signaling)
* Slower, involves changes in gene expression
2) Membrane-initiated steroid signaling
* Faster, includes activation of G proteins and stimulation of protein kinases
Steroid hormones are synthesized by?
favorite it!
Type l receptor
Steroid hormones are synthesized from cholesterol:
- Testosterone, estrogen, and progesterone are the sex steroids, produced by the gonads.
Corticosteroids from the adrenal gland:
- Glucocorticoids—stimulate production of glucose
- Mineralocorticoids—act on the kidney to regulate salt and water balance.
type ll receptors
vitamin A receptor
vitamin D receptor
retinoid
thyroid hormone receptor
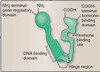
Steroid hormone receptors have three domains:
DNA Binding Domain
Hormone Binding Domain
Gene Regulatory Domain
mnemonics:
Test Everyone Please (damn UMHS whores): Testosterone, estrogen and progesterone.
Got adrenal problems? Get a GLUC and a MK and there wont be a problem! : Adrenal gland: get Glucocoricoids for glucose and Mineralocorticoids for Kidneys.
Thyroid hormone is synthesized from?
Thyroid hormone is synthesized from tyrosine in the thyroid gland.
it is important in development and metabolism. It is transported across the membrane by a carrier protein.
What function does vitamin D3 have?
Vitamin D3 regulates Ca2+ metabolism and bone growth.
Retinoic acid and related compounds (retinoids) are synthesized from?
Retinoic acid and related compounds (retinoids) synthesized from vitamin A play important roles in vertebrate development
Aldosterone
aldosterone a steroid hormone
stimulates renal reabsorption
Cortisol
Cortisol a steroid hormone
- Cortisol increases blood sugar through gluconeogenesis
- anti-inflammatory action
- protein breakdown in muscle
Estrogens
Estrogens is a steroid protein
- Estrogens controls menstrual cycles
- Estrogens promote development of female secondary sex characteristics
What is Progesterone?
Progesterone is a steroid protein
Progesterone causes secretory phase of uterus and mammary glands.
Progesterone causes implantation and maturation of fertilized ovum.
testosterone
testosterone is a steroid protein
- testosterone stimulates spermatogenesis
- testosterone promotes developement of male secondary sex characteristics
- testosterone promotes anabolism (The phase of metabolism in which simple substances are synthesized into the complex materials of living tissue.)
- testosterone promotes masculinization of the fetus
Nuclear-Initiated Steroid Signaling
(favorite it!)
The lipophilic hormones use intracellular gene-specific trancription factors
- Includes steroid hormones, thyroid hormones, retinoic acid and vitamin D
- Receptors are transcription factors
- They bind to DNA promoter elements in genes and alter gene expression
- Steroid hormone receptors often have Zinc finger DNA binding domains
The hormones need to be transported in the blood bound to carrier proteins such as serum albumin, steroid hormone-binding protein or thyroid hormone-binding globulin
Some receptors primarily reside in the nucleus,
Others, such as glucocorticoid receptor are found in the cytosol until the steroid interacts with it
What are the 3 domains seroid receptors?
(favorite it!)
Steroid hormone receptors have three domains:
- Zinc finger DNA Binding Domain
- Hormone Binding Domain
- Gene Regulatory Domain