Block 2 Presentation 2 Transduction part 2 Flashcards
(75 cards)
Signaling through enzyme-coupled cell-surface receptors
Signaling through enzyme-coupled cell-surface receptors

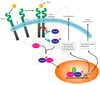
Receptors that are kinases or that bind kinases. The kinase domains are shown in red, and the phosphorylation sites are indicated with red arrows.
A: Tyrosine kinase receptors.
B: JAK-STAT receptors.
C: Serine–threonine kinase receptors.
(pic is located on slide 5 of part 2)
Epidermal growth factor (EGF)
Favorite it!
Epidermal growth factor (EGF) is one of the Receptor Tyrosine Kinases
Signal protein: Epidermal growth factor (EGF)
receptor: EGF receptors
Responses: stimulates cell survival, growth, proliferation, or differentiation of various cell types; acts as inductive signal in development
Receptor Tyrosine Kinases
Receptor Tyrosine Kinases
- The structure of receptor tyrosine kinases
- Single transmembrane polypeptides
- Extracellular ligand binding domain
- Tyrosines on cytosolic tail of receptor
receptor tyrosine kinases phosphorylate tyrosines. they phosphorylate themselves (autophosphorylate) on the cytoplasmic tail.
Additional information (not required)
Figure 24-4 RECEPTOR TYROSINE KINASES. Domain architecture of nine of the 20 families of receptor (R) tyrosine kinases, with ribbon models of several domains. The globular domain of the EphB2 receptor is a β sandwich with a ligand-binding site that includes the exposed loop on the front of this model (PDB file: 1IGY). The extracellular part of the insulin-like growth factor consists of two similar β-helical domains connected by cysteine-rich domains (PDB file: 1IGR). The cytoplasmic kinase domain from the insulin receptor is similar to most known kinases (PDB file: 1IRK). Kinase inserts and C-terminal extensions contain tyrosine phosphorylation sites. Receptor names: EphR, receptor for ephrin, membrane-bound ligands in the nervous system, the largest class of receptor tyrosine kinases; PDGFR, platelet-derived growth factor receptor; FGFR, fibroblast growth factor receptor; VEGFR, vascular endothelial growth factor; Met, receptor for hepatocyte growth factor; TrkA, receptor for nerve growth factor; RET, a cadherin adhesion receptor; Axl, receptor for the growth factor Gas6; EGFR, epidermal growth factor receptor. Domain names: Ig, immunoglobulin; F3, fibronectin-III; CAD, cadherin..)

Achondroplasia
Favorite it!
Example of a dominant negative disorder: Achondroplasia
results from mutation of a receptor tyrosine kinase
it is autosomal dominate disorder.
- Achondroplasia also known as short-limbed dwarfism; charaterized by small stature with short limb, large head, low nasal bridge, prominent forehead, lumber lordosis.
- Incidence is ~ 1 in 10,000
- Pathology
- Failure of cartilage cell proliferation at the epiphyseal plates of the long bones, resulting in failure of longitudinal bone growth, causing short limb.
- Mutation in Fibroblast Growth Factor Receptor 3 (FGFR3) gene;
- ~ 80% of patients are due to new mutation;
- increased risk with late paternal age
- Achondroplasia is a well known, incompletely dominant skeletal disorder of short-limbed dwarfism and large head
- Most achondroplastic individuals have normal intelligence and lead normal lives within their physical capabilities.
- Marriages between two achondroplastic individuals are not uncommon.
- Achondroplasia, an autosomal dominant disorder that often occurs as a new mutation. Note small stature with short limbs, large head, low nasal bridge, prominent forehead, and lumbar lordosis in this typical presentation.
Receptor Tyrosine KinaseThree Major Signaling Branches
Receptor Tyrosine KinaseThree Major Signaling Branches
- Ras/MAPK signaling pathway
- Phospholipase C-gamma signaling pathway
- PI 3-Kinase/AKT signaling pathway
to get one of the above branches going, what needs to occur at the beginning of each branch is that you need a protein in that pathway to interact with the receptor complex. so a member of that pathway will need the ability to dock to a phosphotyrosine.
Regulatory proteins with SH2 domains (Src homology 2 domains) recognize and bind to phosphorylated tyrosines on receptor. so proteins with SH2 domains have the ability to bind to phosphorylated tyrosines.
- This binding stimulates regulatory proteins
Three types of intracellular signaling complexes associated with receptors
Three types of intracellular signaling complexes associated with receptors
- Assemblies help simplify and localize signaling through receptors
- The assembly of signaling complexes depends on various conserved interaction domains, which are found in many intracellular signaling proteins.
(slide 10 for larger pics)

Activation of RTKs
The activation of RTKs

Ligand binds, receptors aggregate, autophophorylation occurs between receptors -Activated receptor tyrosine kinases phosphorylate themselves
Phosphorylated tyrosines on receptor tyrosine kinases serve as?
Phosphorylated tyrosines on receptor tyrosine kinases serve as docking sites for intracellular signaling proteins

Proteins with either SH2 (for SRC homology region) domains, or less commonly, PTB domains (for phosphotyrosine-binding) can bind phosphorylated tyrosines
SH2 domains vary for specificity with neighboring amino acids
SH2 domains vary for specificity with neighboring amino acids
- The SH2 domain is a compact module.
- Each SH2 domain has distinct sites for recognizing phosphotyrosine and for recognizing a particular amino acid side chain, different SH2 domains recognize phosphotyrosine in the context of different flanking amino acid sequences
- This variation allows receptor tyrosine kinases to bind with specificity, multiple proteins containing SH2 domains

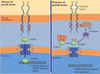
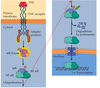
SUBUNIT DIMERIZATION MECHANISM FOR ACTIVATING THE EGF RECEPTOR TYROSINE KINASE
SUBUNIT DIMERIZATION MECHANISM FOR ACTIVATING THE EGF RECEPTOR TYROSINE KINASE

- In the absence of EGF, intramolecular interactions preclude dimerization.
- EGF binding changes the conformation of the extracellular domains allowing dimerization of two receptors, bringing together two cytoplasmic kinase domains.
- Transphosphorylation activates both kinases and creates phosphotyrosine binding sites for SH2 and PTB domains of downstream signal transduction proteins.
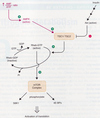
Ras/MAPK Signaling Pathway
Favorite it!
Ras/MAPK Signaling Pathway
- GRB2 (contains SH2 domain) interacts with tyrosine phosphorylated receptor, localizing it to the membrane
- Sos is associated with GRB2 and becomes active (- Sos is a guanine-nucleotide exchange factor)
- Sos stimulates Ras (a monomeric G-protein) to release GDP and bind GTP.
- Ras is now active
- Ras activates RAF (a MAPKKK)
- RAF activates MEK (a MAPKK)
- MEK activates ERK (a MAPK)
- MAPKs phosphorylates transcription factors (such as AP-1)
- Transcription factors induce cells to grow and divide
Ras in inactivated by GTPase activating protein (GAP)

Signal transduction by tyrosine kinase receptors.
Signal transduction by tyrosine kinase receptors.

Signal transduction by tyrosine kinase receptors.
(1) Binding and dimerization.
(2) Autophosphorylation.
(3) Binding of Grb2 and SOS.
(4) SOS is a GEF (guanine nucleotide exchange protein) that binds Ras, a monomeric G-protein anchored to the plasma membrane.
(5) GEF activates the exchange of GTP for bound GDP on Ras.
(6) Activated Ras containing GTP binds the target enzyme Raf, thereby activating it, and a series of kinases known as the MAP kinase pathway.
Ras is a?
Ras is a monomeric GTPase
- Ras is active when bound to GTP
- It is inactive when bound to GDP
- SOS is a GEF which “turns on” Ras
- GAPs “turn off” RAS
- Active RAS binds to RAF

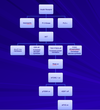
RAS-MAPK Signaling
RAS-MAPK Signaling
- The MAP kinase module activated by Ras
- The three-component module begins with MAP kinase kinase kinase called Raf.
- Ras recruits Raf to the plasma membrane and helps activate it.
- Raf then activates the MAP kinase kinase Mek, which then activates the MAP kinase Erk.
- Erk in turn phosphorylates a variety of downstream proteins, including other protein kinases, as well as gene regulatory proteins in the nucleus.

Oncogenes and signal transduction
Oncogenes and signal transduction

Oncogene proteins act as growth factors (e.g., EGF), growth factor receptors (e.g., ErbB), and intracellular signaling molecules (Ras and Raf). Ras and Raf activate the ERK MAP kinase pathway leading to the induction of additional genes (e.g., fos) that encode potentially oncogenic transcriptional regulatory proteins. Proteins with known oncogenic potential are highlighted with a yellow glow.
Noonan Syndrome
Favorite it!
Noonan Syndrome results from mutations in Ras/MAPK signaling
- Noonan Syndrome is an autosomal dominant condition which is characterized by short stature, distinctive craniofacial features, congenital cardiovascular disease, and other more variable clinical findings such as developmental delay and intellectual disability, bleeding tendencies, lymphatic abnormalities and genitourinary abnormalities.
- Occurs in ~1 in 1,000 individuals
- Often genes encoding Ras/MAPK signaling proteins are mutated
- PTPN11 (Protein Tyrosine phosphatase Shp2)
- KRAS
- SOS
- RAF1
Also note (in the picture): Excessive nuchal skin/webbed neck is sometimes seen in individuals with Noonan syndrome
A 12-year-old female with Noonan Syndrome. Typical webbed neck. Double structural curve with rib deformity

Activation of phospholipase C-g by protein-tyrosine kinases
Favorite it!
Activation of phospholipase C-g by protein-tyrosine kinases
- PLC-γ has SH2 domains that associate with receptor protein-tyrosine kinases.
- Tyrosine phosphorylation increases PLC- γ activity, stimulating hydrolysis of PIP2 to produce InsP3 and DAG.
- InsP3 stimulates calcium release.
- DAG stimulates PKC pathway

EGF RECEPTOR TYROSINE KINASE SIGNALING PATHWAY
EGF RECEPTOR TYROSINE KINASE SIGNALING PATHWAY
- A, Ligand binding changes the conformation of the extracellular domains of the receptor.
- B, Extracellular domains dimerize, bringing together the tyrosine kinase domains of two receptor subunits in the cytoplasm. Direct interactions and transphosphorylation activate the kinases and create specific docking sites for effector proteins with SH2 domains.
- C, Phospholipase C-gamma binds one phosphotyrosine and is activated by phosphorylation to break down PIP2 into diacylglycerol and IP3
- D, A complex of the adapter protein Grb2 and the nucleotide exchange factor SOS binds another phosphotyrosine. SOS catalyzes the exchange of GDP for GTP on the membrane-associated small GTPase Ras. Ras-GTP attracts the cytoplasmic serine/threonine kinase Raf to the plasma membrane.
- E, Raf phosphorylates and activates the dual-function kinase MEK.
- F, MEK phosphorylates and activates MAP kinase.
- G, MAP kinase enters the nucleus and activates latent transcription factors.
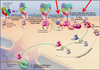
NFAT and Cyclosporin A
Favorite it!
NFAT and Cyclosporin A
- Stimulated T cells trigger PLCg resulting in calcium being released
- Calcium binds and activates calmodulin
- Calmodulin binds and activates a phosphatase called Calcineurin
- Calcineurin dephosphorylates NFAT allowing it to go to the nucleus and stimulate IL-2 gene expression.
- Cyclosporin A : important immunosuppressant used to prevent rejection of transplanted organs
- Works by inhibiting Calcineurin

Receptor tyrosine kinases can also activates?
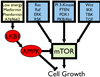
Receptor tyrosine kinases can also activates the PI 3 Kinase/AKT Pathway
- Phosphatidylinositol-3-kinase (PI 3-Kinase) binds phosphorylated tyrosines on receptor and becomes stimulated
- Phosphatidylinositol-3-kinase then phosphorylates phospholipid PIP2 in membrane.
- Regulates cell growth, survival and movement
PI 3 Kinase
- PI 3-Kinase produces lipid docking sites in the plasma membrane
- PI 3-kinase phosphorylates PI (4,5)P2 (PIP2) into PI(3,4,5) P3 (PIP3)
- Intracellular signaling proteins can interact with PIP3 via a specific interaction domain, such as pleckstrin homology domain (PH).
AKT activation
AKT activation
- AKT and the phosphoinositol-dependent kinase (PDK1) bind PIP3
- Another kinase (usually mTOR) phosphorylates AKT, this causes a conformational change that allows PDK1 to phosphorylate AKT
- The activated AKT now dissociates from the plasma membrane and phosphorylates various targets, including the Bad protein.
- Bad is involved with apoptosis.

The PI 3-kinase/Akt pathway
Favorite it!
The PI 3-kinase/Akt pathway
- Ligand binds receptor, receptors aggregate and autophosphorylate each other
- PI 3 Kinase binds phosphorylated tyrosine on receptor and becomes active
- PI 3 Kinase phosphorylates PIP2 to become PIP3
- PDK and AKT (PKB) bind PIP3 molecules
- PDK and mTORC2 then phosporylate and activate AKT (PKB)
- AKT then phosphorylates downstream targets

The PI 3-kinase pathway and cell survival
The PI 3-kinase pathway and cell survival
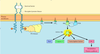
Survival factors such as NGF activate receptor protein-tyrosine kinases, leading to activation of PI 3-kinase and formation of PIP3. PIP3 recruits the protein kinase Akt to the plasma membrane where it is activated as a result of phosphorylation by PDK. Akt then appears to phosphorylate a number of proteins that contribute to cell survival. The targets of Akt that have been implicated in suppression of apoptosis include the Bcl-2 family member Bad, caspase-9, several transcription factors, and the protein kinase GSK-3, which affects cell metabolism and protein synthesis.