Block 2 presentation 3 extracellular structures Flashcards
(19 cards)
Gram-Negative vs Gram-Positive Bacteria
Favorite it!
Gram-Negative vs Gram-Positive Bacteria
- Bacteria are divided into two large classes based on cell wall structure
- Gram-negative bacteria have a dual membrane system, with a thin cell wall in between.
- Gram-positive bacteria have a single plasma membrane, surrounded by a much thicker cell wall.

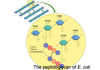
Bacterial Cell Walls
Favorite it!
Bacterial Cell Walls
- The principal component of all bacterial cell walls is a peptidoglycan: linear polysaccharide chains cross-linked by short peptides.
- The cell wall structure also makes bacteria vulnerable to some antibiotics.
- Penicillin inhibits the enzyme DD-transpeptidase that forms the cross-links, preventing cell wall synthesis and bacterial growth.
Two main ways in which animal cells are bound together
Two main ways in which animal cells are bound together
- In connective tissues, the main stress-bearing component is the extracellular matrix.
- In epithelial tissue, it is the cytoskeletons of the cells themselves, linked from cell to cell by anchoring junctions.
- Cell-matrix attachments bond epithelial tissue to the connective tissue beneath it

Extracellular Matrix Functions (some)
Extracellular Matrix Functions (some)
- Adhesion
- Platelet adhesion to each other
- Epithelial attachment
- Filtration
- Glomerular basement membrane (Kidney)
- Structure
- Cartilage
- Bone
- Solubility
Red blood cell antigens
- Migration
- Neural crest formation
- Immune cells
- Sensor mechanisms (all cells)
ECM consists of 3 classes of molecules. what are they?
The Extracellular Matrix of Animal Cells
ECM consists of 3 classes of molecules
- Structural Proteins (collagen, elastins)
- Protein-polysaccharide complexes (proteoglycans)
- Adhesive glycoproteins (fibronectins)

Collagen
Collagen
- Collagens are responsible for the strength of the extracellular matrix
- Two Defining characteristics of collagen: a rigid triple helix and unusual amino acid composition (high % glycine)
- The stability of collagen fibril is reinforced by hydrogen bonds that involve hydroxyl groups of hydroxyproline and hydroxylysine residues in the a chain
- Collagens form triple helices: three polypeptide chains are wound around one another in a ropelike structure.
- The triple helix domains consist of repeats of the amino acid sequence Gly-X-Y (a glycine in every 3rd position).
- Glycine is the smallest amino acid, and allows polypeptides to pack closely together.
- Proline is frequently found in the X position and hydroxyproline in the Y position; they stabilize the helices.
(pic slide 12)

Formation of hydroxyproline
Favorite it!
Formation of hydroxyproline
- Hydroxyproline is formed in the Endoplasmic Reticulum by modification of proline in collagen polypeptide chains.
- Enzyme reaction requires Vitamin C
- Hydroxyl groups are thought to stabilize the triple helix by forming hydrogen bonds.

Collagen fibrils
Collagen fibrils
- After being secreted, these collagens form collagen fibrils in which the triple helical molecules are associated in regular staggered arrays.
- Covalent cross-links between the side chains of lysine and hydroxylysine residues further strengthen the fibrils.
- Fibrils can come together to form collagen fibers, which can be several µm in diameter.
- Other types of collagen do not form fibrils.
- Fibril-associated collagens bind to collagen fibrils and link them to one another and to other matrix components.
The many types of collagens
favorite it
The many types of collagens
type 1= most connective tissues
- Collagen I: skin, tendon, vascular ligature, organs, bone (main component of bone)
type 2= cartiliage and vitreous humor
- Collagen II: cartilage (main component of cartilage)
type 3= extensible connective tissue
- Collagen III: reticulate (main component of reticular fibers), commonly found alongside type I.
type 4= basal laminae (network filament)
- Collagen IV: forms bases of cell basement membrane
additional info
- 40 different kinds of a chain combine to form at least 28 different types of collagen
- Types I, II and III are the most common (type I ~ 90% of collagen)
- Striated bands reflect the regular offset manner in which triple helices associate laterally to form fibrils
Glycogen synthesis
favorite it!
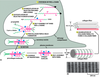
transcription occurs in the nucleus so the collagen alpha chain will be transcribed.
the mRNA will go out into the cytosol. translation will start but does not go into completion because SRP will stop it by binding to the signal peptide.
SRP will cause the ribosome to dock at the cytosolic side of rough ER and will allow for continue synthesis of the alpha chain.
in the lumen of the rough ER the alpha chain, which include the signal peptide/sequence by signal peptidylase to turn pre-pro collegen to pro collagen.
in the lumen of the rough ER you get the hypdroxylation of proline and lysine. Vitamin C is critical for that.
you will have glycosylation begin in the lumen of the rough ER on hydroxylated residues and form your triplehelix.
at this point vesicles budding off your golgi will be sent to the plasma membrane where the immature pro collagen triple helixes leaves the cell through exocytosis to get outside of the cell. this is where the ends of the immature collagen is removed by N and C pro-collagen peptidases, once you chop off the frayed ends, now you have mature collagen.
The mature pro collagen triplehelix spontaneously self assemble into fibrils (immature collagen couldnt do this). the fibrils have to be cross linked this point, lyso-oxisidase enzyme helps collagen crosslink in the presence of copper.
don’t confuse!
lyso-oxidase= found outside of the cell functioning in crosslinking of collagen. it is copper dependant.
lyso-hydroxidase= found in the lumen of the rough ER where its job is to hydroxilate lysine.
additiona info from slides
- Procollagen triple helix formed in the ER
- Hydroxyproline and hydroxylysine formed
- Procollagen secreted from cell
- In the intercellular space, procolloagen peptidase converts procollagen to collagen in the intercellular space. The enzyme remove amino acids from both the N- and C- terminal ends
- The resulting collagen molecules spontaneously associate and polymerize to form mature collagen fibrils, which then assemble laterally into fibers
Intracellular processes:
- Individual collagen alpha chains are synthesized in the RER
These proteins are sometimes called pre-procollagen chains)
(Note: lysines and prolines are also hydroxilated at this step.)
(Note: the initial alpha chain synthesis contains terminal non-collagenous domains)
- Vesicle transport of pre-procollagen chains to the Golgi
- Triple helical formation of pre-procollagens to make procollagen.
- Transport of procollagen outside of cell.
Extracellular processes:
- Cleavage of non-collagenous domains to form tropocollagen.
- Tropocollagen assembles into fibrils
- Extracellular enzymes covalently bind hydroxylysines of different tropocollagens to stabilize fibrils.
Diseases associated with collagen
Favorite it!
Diseases associated with collagen

Scurvy
Ehlers-Danlos syndromes
Osteogenesis Imperfecta
Menkes disease
Osteogenesis Imperfecta
Favorite it!
Osteogenesis Imperfecta
The characteristic features of **Osteogenesis Imperfecta vary greatly from person to person–even among people with the same type of OI, and even within the same family–and not all characteristics are evident in each case.** The general features of the four recognized types of OI, which vary in characteristics and severity, are as follows:
Osteogenesis Imperfecta - Type I
Favorite it!
Osteogenesis Imperfecta - Type I
- EFFECT TYPE I COLLAGEN
- Most common and mildest type of OI.
- Bones predisposed to fracture. Most fractures occur before puberty.
- Normal or near-normal stature.
- Loose joints and low muscle tone.
- Sclera (whites of the eyes) usually have a blue, purple, or gray tint.
- Triangular face.
- Tendency toward spinal curvature.
- Bone deformity absent or minimal.
- Brittle teeth possible.
- Hearing loss possible, often beginning in early 20s or 30s.
- Collagen structure is usually normal, but the amount is less than normal.
- unable to move the immature collagen from to the golgi and beyond after glycosylation in the RER because no triplehelical immature collagen was formed.
Type I collagen: bones, tendon, ligaments, detin (teeth). associated disease. osteogenesis imperfecta. Ehlers-danlos syndromes (which also involves collagen type 3)
type II collagen: cartilage, nucleus pulposus.
Type III collagen: skin ehlers-Danlos syndrome. (which also involves collagen type 1)
Type IV collagen: basement membrane.
Osteogenesis Imperfecta - Type II
Favorite it!
Osteogenesis Imperfecta - Type II
- Most severe form.
- Frequently lethal at or shortly after birth, often due to respiratory problems.
- In recent years, some people with Type II have lived into young adulthood.
- Numerous fractures and severe bone deformity.
- Small stature with underdeveloped lungs.
- Collagen is improperly formed.
Autosomal dominate disorder as well. But in diseases where theres a high percentage of death in babies who have it… than it is caused by a new mutation, which is seen in Osteogenesis Imperfecta
Caused by mis sense mutation on a glycine residue more towards the carboxy terminal of the alpha end. Triple helix formation normally starts at the carboxyl terminal end of the alpha chain
Ehlers-Danlos syndromes
Favorite it!
Ehlers-Danlos syndromes
- An array of disorders that involve collagen and connective tissue.
- (Six types and multiple subtypes, Types I-VI)
- Commonly associated with loose or hypermobile joints and hyperelastic/ hyperflexible joints

Scurvy
Favorite it!
Scurvy
- Subperiosteal hemorrhages lead to pain in bones and joints.
- Petechial hemorrhages, ecchymoses, and purpura are common.
- Perifollicular hemorrhages in the skin
- In advanced cases, swollen, bleeding gums are a classic finding.
- Alveolar bone resorption results in loss of teeth.
- Poor wound healing.
- Anemia may result from prolonged bleeding,
- impaired iron absorption, or associated folic acid deficiency.
- Patients have difficulty walling off infections to form abscesses, so that infections spread more easily.
- In children, vitamin C deficiency leads to growth failure and collagen-rich structures such as teeth, bones, and blood vessels develop abnormally.
At risk: Infants, elderly men, alcoholics, smokers
- Vitamin C is easily lost via cooking, sensitive to heat
Scurvy
- Deficient for 20-40 days
- Swollen, bleeding gums, joints, loose teeth, pinpoint hemorrhages around hair folicles (petechiae), gums, nails, soreness and stiffness of joints & lower extremities, slow wound healing, anemia, fatigue
Rebound scurvy
- Immediate halt to excess vitamin C supplements

Menkes syndrome
Favorite it!
Menkes syndrome
- Menkes syndrome is an inborn error of metabolism that markedly decreases the cells’ ability to absorb copper.
- The disorder causes severe cerebral degeneration and arterial changes, resulting in death in infancy. The disease can often be diagnosed by looking at a victim’s hair, which appears to be both whitish and kinked when viewed under a microscope.
- Menkes’ disease is transmitted as an X-linked recessive trait.
- Menkes disease is a disorders of copper transport caused by mutations in the copper-transporting ATPase gene (ATP7A).
- The Lysyl oxidase enzyme is affected (dependent on copper). It is an extracellular enzyme that acts to establish crosslinking
- Sulfhydril oxidase that crosslinks keratin also requires copper.
- Sufferers can not transport copper, which is needed by enzymes involved in making bone, nerve and other structures. A number of other diseases, including type IX Ehlers-Danlos syndrome, may be the result of allelic mutations (i.e. mutations in the same gene, but having slightly different symptoms).
- Menkes disease is suspected in male infants who develop hypotonia, failure to thrive, and seizures at six to ten weeks of age. Shortly thereafter, hair changes become manifest; the scalp and (usually) eyebrow hair is short, sparse, coarse, twisted, and often lightly pigmented (white, silver, or gray).
The hair is shorter and thinner on the sides and back of the head. The hair can be reminiscent of steel wool cleaning pads.
- Light microscopic hair analysis reveals pili torti (hair shafts twisting 180°), trichoclasis (transverse fracture of the hair shaft), and trichoptilosis (longitudinal splitting of the hair shaft). Due to flattening of the normal cylindrical structure, the periodicity of the twisting in pili torti is different from naturally curly hair.
Specific clinical features include:
- Distinctive facial features (jowly appearance with sagging cheeks)
- Pectus excavatum (midline depression in the bony thorax)
- Skin laxity particularly on the nape of the neck and trunk
- Umbilical or inguinal herniae
- Hypotonia, neurodevelopmental delays, and failure to thrive, typically manifest at three to six months of age
What are the two proteins that start synthesis in the cytosol, is halted by SRP, goes to the ER and can be synthesized to completion in the ER?
Insilin and collagen!
what do they have in common you ask?
they both have to be secreted!
TGF-β
TGF-β found in the serine threonine pathway (enzyme coupled receptor) and elastin.
Fibrillin can influence cell signaling because it influences TGF-β


