Brain Flashcards
(86 cards)
Ventricular System and Hydrocephalus
The normal ventricular system consists of the:
- Third ventricle
- Intervertericular formane - connects the 3rd ventricle to the lateral ventricles
- Mesencephalic aqueduct - connect the 3rd ventrcle to the 4th ventricla
- Fourth ventricle
- Connects with the central canal
- Llateral ventricles
The third ventricle communicates with the lateral ventricles via the interventricular foramina, and the mesencephalic aqueduct connects the third and fourth ventricles. Caudally, the fourth ventricle communicates with the central canal.
Cerebrospinal fluid (CSF):
- Produced by the choroid plexus, located on the floor of the lateral ventricles and on the dorsal margins of the third and fourth ventricles.
- CSF circulates throughout the ventricular system and exits into the subarachnoid space through the lateral apertures of the fourth ventricle.
- CSF is resorbed primarily through arachnoid villi that extend into the dural venous sinuses and secondarily through lymphatic drainage of the meningeal sheaths surrounding nerve roots.
Hydrocephalus
Hydrocephalus is defined as an abnormal distension of all or part of the ventricular system with CSF. Ventricular distension is typically caused by constant or intermittent increased hydrostatic pressure. The term hydrocephalus denotes the anatomic status of the ventricular system rather than an underlying cause. Hydrocephalus can be developmental or acquired and can result from:
- Obstruction of CSF drainage
- Impaired CSF resorption
- CSF overproduction.
The latter two forms are referred to as communicating or nonobstructive hydrocephalus. Use of a related term, ventriculomegaly, may be more appropriate when passive ventricular enlargement occurs as a result of diminished brain parenchyma volume (also known as hydrocephalus ex vacuo). Normal CSF has a density near that of pure water and will therefore have a HU value of close to 0 on unenhanced CT images and will be hypoattenuating to surrounding brain parenchyma. On unenhanced MR images, normal CSF will appear T1 hypointense and T2 hyperintense to brain parenchyma and will have no or low signal on water‐nulling sequences, such as FLAIR. In patients with abnormal CSF due to hemorrhage, inflammation, or neoplasia, signal intensity may be significantly increased on T1 and pure water‐nulling sequences depending on cellular and macromolecular content.
Congenital hydrocephalus
- Congenital hydrocephalus occurs predominantly in brachycephalic and toy breeds.
- In some instances, mechanical obstruction from such entities as mesencephalic duct stenosis or Chiari malformation explains the presence of hydrocephalus; in other cases, no underlying cause is recognized.
Obstructive hydrocephalus
- Obstructive hydrocephalus may be due to intraluminal or extraluminal masses or other lesions that impair CSF flow within the ventricular system.
- The underlying causes of obstructive hydrocephalus vary widely, as do the imaging features of the ventricular system.
- Depending on the source and location of obstruction, hydrocephalus may be uniform or regional.
- Obstruction originating in or caudal to the fourth ventricle will tend to produce uniform ventricular distension, whereas obstruction in the third or lateral ventricles or in the interventricular foramina may produce asymmetrical, regional, or focal ventricular distension
Communicating (nonobstructive) hydrocephalus
Impaired CSF resorption
- Impaired CSF resorption is thought to occur with diminished resorptive capacity of the arachnoid villi.
- Postulated causes include intraventricular hemorrhage and ventriculitis with cells or debris causing obstruction of the valvular flow mechanism of individual villi.
- Chronic hydrocephalus also appears to diminish resorptive function of the villi.
Hydrocephalus from CSF overproduction
- Hydrocephalus from overproduction of CSF is thought to occasionally occur in some patients with functional choroid plexus tumors in which the abnormally high rate of CSF production exceeds the rate of resorption
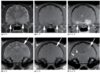
Normal Ventricles (Canine)

7y FS French Bulldog.
- The normal T1 hypointense appearance of the third ventricle surrounding the interthalamic adhesion (a,b: arrows), the lateral ventricles (b: arrowheads), and the fourth ventricle (a: arrowhead).
- The mesencephalic duct is also seen as a thin, curvilinear, hypointense communication between the third and fourth ventricles (a).
- Normal cerebrospinal fluid appears hyperintense on T2 images (c) and profoundly hypointense on FLAIR images (d).

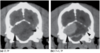
Ex vacuo Hydrocephalus (Canine)
akak “ventriculomegaly”

4y MC Maltese recovering from a penetrating head injury (dog bite) 6 months previously.
- Posttraumatic cortical atrophy of the left cerebral hemisphere results in passive expansion of the left lateral ventricle to fill the potential space.
- This dog also has evidence of more generalized ventriculomegaly.
- Discontinuity of the overlying parietal bone from previous fracture is evident on both the CT and MR images.

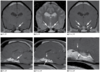
Hydrocephalus with Abnormal Cerebrospinal Fluid (Feline)

2y MC Domestic Shorthair with signs of a C1–C5 myelopathy.
- Bilateral lateral ventriculomegaly is seen on all MR sequences.
- Cerebrospinal fluid (CSF) is uniformly T2 hyperintense (a), but CSF in the right lateral ventricle is moderately intense compared to the low‐intensity signal within the left lateral ventricle on FLAIR (b) and T1 (c) images, indicating compartmentalization and cells or macromolecules contaminating the CSF in the right lateral ventricle.
- Thickening and intense enhancement of the right lateral ependymal lining is evident on the contrast‐enhanced T1 image (d).
A diagnosis of feline infectious peritonitis was based on cerebro- spinal fluid cytology and coronavirus titers.

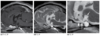
Congenital Hydrocephalus (Canine)

4y MC English Bulldog with intermittent seizures.
- Marked generalized hydrocephalus is seen on all image sequences.
- Although enlargement of the lateral ventricles is most striking, third ventricular dilation is also evident, which is best appreciated on the sagittal T1 image (a: asterisk).
- Ventriculomegaly was thought to be breed related.

Obstructive Hydrocephalus (Feline)

7mo MC Domestic Shorthair with multifocal central nervous system signs.
- An obstructive mass was detected in the caudal brainstem and in the spinal cord at the level of C1 (a: arrowhead).
- Marked generalized ventriculomegaly is evident on all images, and distension of the fourth ventricle and mesencephalic duct is particularly prominent (a: arrows).
A diagnosis of feline infectious peritonitis was based on cerebrospinal fluid cytology and coronavirus titers.

Obstructive Hydrocephalus (Canine)

5y MC Labrador Retriever with hydrocephalus affecting the left lateral ventricle.
- A small contrast‐enhancing mass is present on the ventral margin of the left lateral ventricle (a,d: arrowhead).
- The left lateral ventricle is markedly distended, and there is a thin rim of hyperintensity, best seen on the FLAIR image (b: arrows), thought to represent transependymal interstitial edema.
A choroid plexus carcinoma involving the floor of the left lateral ventricle and causing partial foraminal obstruction was confirmed on postmortem examination.

Presumptive Overproduction Hydrocephalus (Canine)

6y FS Labrador Retriever with clinical signs of weakness and obtundation.
- A large, well‐defined mass is present within the third ventricle (a–d: arrow), and generalized hydrocephalus is present (a–d: arrowheads).
A solitary third ventricle choroid plexus carcinoma was confirmed on postmortem examination. There was no evidence of overt obstruction, which led to a presumptive diagnosis of overproduction hydrocephalus.

Brain Edema

Brain edema may result from a wide array of causes, which can be divided into the four principal forms listed in Table 2.2.1.1–3 Clinically, multiple forms of brain edema can occur simultaneously, and often the predominating form depends on the inciting cause as well as the time course of the disease.
Whether intracellular or extracellular, edema appears mildly to moderately hypoattenuating to normal brain parenchyma on CT images and T1 hypointense and T2 hyperintense on MR images. Because edema fluid is distributed within a microenvironment of cells and macromolecules, it will also appear hyperintense on FLAIR and other pure water‐nulling sequences.
Cytotoxic edema
- Cytotoxic edema occurs as a result of ischemia resulting in cell membrane Na/K pump dysfunction, increased intracellular fluid volume, and cell swelling.
- Because of the underlying cause and the intracellular nature of this form of edema, white and gray matter may both be affected, and the distribution of edema roughly conforms to the geographic distribution of ischemia
- In most instances, cytotoxic edema occurs in combination with vasogenic edema.
- Diffusion‐weighted imaging has been used to discriminate between the two forms following acute episodes of ischemia, with reduced apparent diffusion coefficient (ADC) intensity reflecting predominantly cytotoxic edema.
Vasogenic edema
- Vasogenic edema occurs because of a disruption of the tight junctions of the blood–brain barrier, resulting in extravasation of high‐protein fluid into the brain.
- Vasogenic edema is extracellular, so it tends to preferentially accumulate in white matter, which has a sparser cellular density and therefore more potential space for fluid distribution compared to highly cellular gray mat ter.
- Depending on the initiating cause and volume of fluid, edema can distribute widely
Interstitial or hydrocephalic edema
- Interstitial edema most often occurs in association with obstructive hydrocephalus when intraventricular pressure increases, causing transependymal CSF migration into adjacent brain parenchyma.
- As a result, hydrostatic edema preferentially occurs within periventricular parenchyma and is extracellular.
- Unlike vasogenic edema, interstitial edema fluid is a transudate containing little in the way of cells or macromolecules.
Osmotic edema
- Osmotic edema occurs rarely and is caused by reduced plasma osmolality resulting from water intoxication, hemodialysis, or metabolic disorders that reduce plasma sodium or glucose concentration.
- The imbalance in brain extracellular fluid osmolality and plasma osmolality results in a fluid shift to the brain leading to formation of extracellular edema.

Cytotoxic Edema (Canine)

3y FS Dachshund with right‐sided cerebellar infarction.
- There is a well‐circumscribed geographic region of FLAIR and T2 hyperintensity involving the right cerebellum (b,c: arrow).
- The T2 hyperintensity is due, in part, to intracellular cytotoxic edema resulting from cell hypoxia.
- The lesion distribution coincides with the tissue volume normally perfused by the right rostral cerebellar artery.

Vasogenic Edema (Canine)

3y MC Basset Hound with aspergillosis involving the frontal sinus and forebrain. This unenhanced CT image is caudal to the primary lesion.
- Marked, diffuse hypoattenuation is evident involving the white matter of the right cerebral hemisphere because of the presence of vasogenic edema.
- Although edema is recognized on CT images, it may be less conspicuous than on corresponding MR images.

Vasogenic Edema (Canine)

Adult dog of unknown age and gender with a large left frontal lobe meningioma. This image is at a level caudal to the mass.
- Marked, diffuse hyperintensity is evident involving the white matter of the left cerebral hemisphere, representing vasogenic edema.
- There is also diffuse volume expansion of the white matter associated with prominent right‐sided midline shift.

Interstitial Edema (Canine)

6y FS Toy Poodle with a caudal fossa meningioma causing obstruction of the ventricular system (a). Images b and c are at the level of the rostral horns of the lateral ventricles.
- The thin, hyperintense rim surrounding the rostral horns of the lateral ventricles on the FLAIR image (c: arrowheads) represents transependymal migration of cerebrospinal fluid to the periventricular extracellular fluid space due to increased intraventricular hydrostatic pressure.

Developmental Disorders of the Brain
Anomalous development of the brain
Brain malformation in the dog and cat can be induced by trauma, toxins, inflammatory disorders, serendipitous in utero aberrations, and genetic defects. Brain development can be broadly divided into five progressive stages:
- Dorsal induction—ventral induction
- Neuronal proliferation
- Differentiation and histogenesis
- Neuronal migration
- Myelination
Anomalies can arise during any one of these stages, and the type of anomaly will reflect the predominant development activity at the time.
Most significant anomalies are rarely imaged with CT or MRI since many patients die or are euthanized early in life. Although classification schemes for developmental brain anomalies vary widely, we have chosen to organize this section into:
- Hindbrain herniations and malformations
- Diverticulation and cleavage disorders
- Malforma tions of cortical development
- Nonneoplastic cysts
Hindbrain herniations and malformations:
Chiari-like malformation
- Chiari‐like malformation is due to reduced volume of the caudal cranial fossa, resulting in cerebellar to caudal cranial fossa volume mismatch. The disorder occurs primarily in Cavalier King Charles Spaniels, but other small and toy breed dogs can be affected. The reduced caudal fossa volume results in crowding and repositioning of the cerebellum, which may sometimes encroach on or herniate through the foramen magnum. Cerebellar crowding also causes extramural compression of the fourth ventricle and central canal, which leads to obstructive hydrocephalus and syringohydromyelia.
- Clinical signs include pain, positional pain, hyperesthesia, and neurologic deficits, but severity of clinical signs correlate poorly to imaging findings.
- On CT images, the caudal fossa will appear smaller than normal, which may be best appreciated on sagittally reformatted images. Obstructive hydrocephalus and syringohydromyelia may also be seen. Similar features will be seen on MR images, and a sagittal T2 sequence is often best for detecting ventricular and central canal distension and for recognizing cerebellar displacement and foraminal herniation
Cerebellar hypoplasia
- Cerebellar hypoplasia has been reported in cats as a sequela to in utero parvovirus infection. The disorder has also been reported in dogs, but a distinction between cerebellar hypoplasia and cerebellar atrophy from degeneration may be challenging antemortem. On MR images in people, the cerebellum is small and may appear to float in an expanded subarachnoid space. The number of folia may also be reduced. Similar gross features have been reported in domestic animals.
Cerebellar vermian hypoplasia
- Cerebellar vermian hypoplasia is a rare disorder in which the cerebellar vermis is hypoplastic or absent. In some patients, the cerebellar hemispheres and flocculus may also be involved and the caudal cranial fossa can be enlarged. The anomaly is analogous to Dandy–Walker syndrome in people.
- On unenhanced CT images, the cerebellar vermis is hypoplastic or absent, leaving a potential space filled by an expanded fourth ventricle, which is hypoattenuating compared to adjacent brain parenchyma.
- Unenhanced MR imaging features are reported to be similar, with enlargement of the fourth ventricle appearing T1 hypointense and T2 hyperintense. Concurrent hydrocephalus has been reported in one dog.
Diverticulation and cleavage disorders
- Diverticulation and cleavage disorders include complex anomalies, such as holoprosencephaly and septo‐optic dysplasia, that occur early in development and involve not only the brain but may affect the face, cranial nerves, and pituitary gland as well.
- Such anomalies are not well described in domestic animals since most affected animals likely die early in life. On both CT and MR images, these disorders will vary depending on the nature and severity of the anomaly.
Malformations of cortical development
- Malformations of cortical development represent a diverse group of developmental anomalies, including microencephaly, pachygyria–polymicrogyria, lissen cephaly, and schizencephaly.
- These anomalies may feature variable and often reduced brain volume, cortical convolution anomalies, and cortical clefts.
- On both CT and MR images, the appearance of these disorders will vary depending on the nature and severity of the anomaly, although disruption of the normal contours of the cortex is a consistent feature
Nonneoplastic cysts
Arachnoid cysts
- Intracranial arachnoid cysts arise from the arachnoid membrane surrounding the brain, do not communicate with the ventricular system, and are thought to be primarily developmental (although acquired cysts are suspected to also occur).
- Young, small‐breed brachycephalic dogs are most frequently affected, although cysts have also been reported in other canine breeds and in cats. Arachnoid cysts most commonly arise from the quadrigeminal cistern but will occasionally occur in other locations.
- Uncomplicated arachnoid cysts have a thin unicameral membrane, contain cerebrospi nal fluid, and conform to the margins of adjacent structures. Although many quadrigeminal arachnoid cysts are clinically silent, large cysts can produce cerebellar and occipital lobe compression leading to development of neurologic clinical signs. The presence of intracystic hematomas has been reported and may lend credence to the thought that some arachnoid cysts are traumatic in origin, as described in people.
- On unenhanced CT images, uncomplicated intracranial arachnoid cysts have well‐defined margins, contain fluid isoattenuating to cerebrospinal fluid, and do not contrast enhance.
- On MR images, cysts are clearly extraaxial, contain fluid isointense with CSF, and do not contrast enhance.
- Arachnoid cysts containing blood or organizing hematomas may have variable attenuation on CT and variable T1 and T2 signal intensity on MRI.
Epidermoid and dermoid cysts
- Epidermoid and dermoid cysts are rare and result from aberrant ectodermal cell migration and entrapment during neural tube closure. The most common locations are the fourth ventricle and cerebellopontine angle. Clinical signs may result from obstructive hydrocephalus.
- Epidermoid cysts consist primarily of desquamated skin cells. These masses appear hypoattenuating to adjacent brain on unenhanced CT images and are T1 hypointense and T2 hyperintense on unenhanced MR images. Epidermoid cysts would not be expected to enhance unless ruptured, producing a peripheral inflammatory response
- Dermoid cysts are more complex, containing hair follicles and sebaceous material that appear hypoattenuating on unenhanced CT images and T1 and T2 hyperintense on unenhanced MR images because of the lipid content. Fat‐suppressed T1 sequences may be used to null the lipid signal to better characterize the lesion. Similar to epidermoid cysts, dermoid cysts would not be expected to enhance unless ruptured, producing a peripheral inflammatory response.
Chiari‐like Malformation (Canine)

5y F Cavalier King Charles Spaniel with intermittent neck pain and C1–C5 myelopathy. Image c is a magnification of image b.
- Malformation of the occipital bone (a,b: arrow) results in reduced caudal cranial fossa volume.
- Mild enlargement of the third and fourth ventricles (a) and sacculated cervical syringohydromyelia (d) are evident.
- Herniation of the cerebellum through the foramen magnum is best seen on sagittal T2 images (b,c: arrowhead).

Small Cerebellum – Probable Cerebellar Hypoplasia (Canine)

3.5mo M Cocker Spaniel cross with neurologic signs referable to the cerebellum.
- The cerebellum is small, and the surface contours appear unusually well defined because of increased cerebrospinal fluid volume surrounding the cerebellar folia.
- The fourth ventricle and cerebellomedullary cistern are also larger than expected.
This diagnosis was not confirmed by biopsy or postmortem examination.

Presumptive Cerebellar Vermian Hypoplasia (Canine)

Adult dog of unknown age and unknown clinical signs. The representative transverse images are at the level of the rostral (b,d) and caudal (c,e) cerebellum.
- The volume of the caudal fossa is larger than expected, and the cerebellum is markedly reduced in size (a: arrow).
- The fluid surrounding the cerebellum within the caudal fossa is likely compartmentalized cerebrospinal fluid within a grossly distended cerebellopontine cistern.
- Both cerebellar hemispheres are hypoplastic (b–e: asterisks), and the central cleft (c: arrow) is indicative of aplasia of the caudal aspect of the cerebellar vermis.
- There is free communication (e: black double‐headed arrow) of the fourth ventricle (e: small arrow) with a markedly enlarged cerebellomedullary cistern (e: large arrow).
Although not confirmed by postmortem exam, the constellation of imaging findings is characteristic of Dandy–Walker syndrome with cerebellar vermal hypoplasia/aplasia described in people.

Lissencephaly (Canine)

Lhasa Apso of unknown age.
- The normally complex surface convolutions of the cortical gyri and sulci are absent and mild, generalized hydrocephalus is present (a–d).
- In addition, there is a striking lack of white‐matter architectural detail (c).
- This dog also has a quadrigeminal arachnoid cyst (d: arrow).

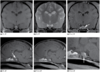
Complex Cortical Developmental Anomaly (Feline)

4mo F Domestic Shorthair with obtundation and rotary nystagmus. Representative transverse and parasagittal images reveal a complex brain anomaly that includes profound hydrocephalus and abnormal cortical and corpus callosum development (a–c). Postmortem examination documented hydrocephalus, hypoplasia of the corpus callosum, cortical gyral malformation, and pachygyria (d).

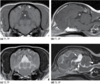
Arachnoid Cyst (Canine)

2y FS Maltese with unlocalized pain without neurologic deficits. Images a–c are representative transverse images of the brain at the level of the parietal lobes (a), occipital lobes (b), and cerebellum (c).
- Moderate, symmetrical lateral ventriculomegaly is present (a).
- A large, fluid‐attenuating arachnoid cyst (b–d: asterisk) arises from the quadrigeminal cistern and is bounded ventrally by the tectum (d: large arrow) and cerebellum (d: arrowhead), rostrally by the corpus callosum (d: small arrow), and dorsally by the tentorium cerebelli (not seen).
- The cyst is predominantly subtentorial, and it displaces and compresses the cerebellum ventrally.

Arachnoid Cyst (Canine)
MR

3y MC Shih Tzu with tetraparesis. The MR examination was performed to fully evaluate a diagnosed occipitoatlantoaxial malformation. Transverse images (a,c) are at the same level at the occipital lobes.
- A well‐defined arachnoid cyst with pure‐water signal characteristics arises from the quadrigeminal cistern (a,b: asterisk) and is bounded ventrally by the tectum (d: large arrow) and cerebellum (d: arrowhead), and rostrally by the corpus callosum (d: small arrow).
- Focal spinal cord narrowing and parenchymal T2 hyperintensity are also evident on image d, associated with the occipitoatlantoaxial malformation.
Hemorrhage

Staging Hemorrhage
The information in Table 2.4.1 is extracted from the human MRI literature, but it should approximate hemorrhage‐staging patterns in veterinary patients using unenhanced spin‐echo T1 and T2 intensities. In addition, T2* gradient echo sequences can be used to detect signal void from susceptibility of blood degradation products at most stages, though the susceptibility “bloom” sometimes overstates the actual hemorrhage volume.
Because hematoma density is greater than that of normal brain parenchyma, acute to subacute hemorrhage is hyperattenuating compared to brain parenchyma on unenhanced CT images. Density gradually reduces to become isoattenuating with brain parenchyma over many days to weeks.
Extraaxial hemorrhage
Extraaxial hemorrhage is classified as epidural, subdural, or subarachnoid, although in our experience subarachnoid hemorrhage is less frequently recognized.
Epidural hematoma
Epidural hematomas are most often traumatic in origin, arise in the potential space between the cranium and the dura mater, and typically occur from meningeal arterial hemorrhage. Epidural hematomas are described as having a characteristic biconvex or lenticular shape on cross‐sectional images. Acute epidural hematomas appear hyperattenuating to brain parenchyma on unenhanced CT images and will have variable unenhanced T1 and T2 intensity on MR images, depending on the age of the hematoma.
Subdural hematoma
Subdural hematomas are usually traumatic in origin, arise in the potential space between the dura mater and the arachnoid membrane, and typically occur as the result of venous sinus hemorrhage. Subdural hematomas are crescent shaped, conforming to the convex surface of the brain. Acute subdural hematomas will appear hyperattenuating to brain parenchyma on unenhanced CT images with a gradual reduction in density over time. They will have variable unenhanced T1 and T2 intensity on MR images, depending on the age of the hematoma.
Subarchnoid hemorrhage
Head trauma may cause bleeding into the subarachnoid space. Acute subarachnoid hemorrhage will appear hyperattenuating and will generally conform to the convolutions of the cerebral cortex and the cisterns on unenhanced CT images. Acute subarachnoid hemorrhage will appear T1 isointense and T2 and FLAIR hyperintense on MR images with a distribution similar to that seen on CT. Intensity patterns will change with chronicity.
Brain contusion and hemorrhage
Imaging features of brain contusion depend on the combination of edema and hemorrhage in the affected brain parenchyma. Edematous regions will appear hypoattenuating and focal areas of hemorrhage will appear hyperattenuating on unenhanced CT images . Edema will appear T1 hypointense and T2 hyperintense on MR images with hemorrhagic regions having a T2* signal void and an otherwise variable appearance depending on duration since trauma. Edema and hemorrhage will increase brain parenchymal volume, which can lead to:
- midline shift
- ventricular compression
- sulcal and gyral effacement
- brain herniation.
Magnetic resonance angiography, diffusion and perfusion weighted imaging, and diffusion tensor imaging can all be used to further characterize the extent of injury.

Vascular Disorders
Most infarctions are arterial in origin, and although stroke from venous thrombosis is described in people, there are few comparable reports in veterinary medicine. The rostral and middle cerebral and the striate and rostral cerebellar arteries are the most commonly involved, and infarcts involving the cerebrum, thalamus/midbrain, and cerebellum have been reported.
Infarcts are described as territorial when they involve a major intracranial vessel and lacunar when smaller penetrating vessels are obstructed. Underlying causes for stroke include:
- Atherosclerosis
- Hypertension
- Diabetes in people
**although these have not been confirmed as predisposing factors in veterinary patients.
Hematomas

Hematomas will generally appear as a hyperattenuating mass on unenhanced CT images, and there may be evidence of contrast enhancement if active bleeding (acute) or neovascularization (chronic) is present. MR imaging features will generally follow the scheme outlined in Table 2.4.1, although age can be ambiguous when multiple bleeding episodes occur over time. Secondary features of mass effect may include surrounding edema, midline shift, ventricular displacement and compression, and sulcus and gyrus effacement on both modalities.