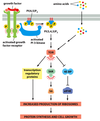
Cell cycle Flashcards
(40 cards)
What are the phases of interphase?
G1, S, G2
What are the phases M phase?
mitosis and cytokinesis
Describe several methods to study the cell cycle (including model organisms)
model organisms:
- zebrafish (BrdU and antibody)
- rat fibroblasts (are round when in mitosis)
- budding yeast (bud off when mitosing)
- fission yeast (length is indicative of cell cycle stage)
- flow cytommetry can measure the content of cells to determine the stage of the cell cycle
How do we know the cell cycle control proteins are highly conserved?
you can place proteins from one vertebrate into another and oftentimes the cell cycle functions as normal.
What is the restriction point?
It is the point in the cell cycle where DNA replication begins and the cell can not go back.
What is the purpose of the cell cycle checkpoints?
- surveillance systems
- quality control mechanisms of the genome
- required for accurate transmission of genetic information
What is the engine of the cell cycle?
cyclin-dependent kinases
- conserved throughout evolution
- phosphorylate serine or threonine residues of target proteins at K/R S/T X K/R consensus sequence
List the major cyclins and Cdks of the vertebrate cell cycle.

Describe the four regulatory mechanisms of Cdks.
- transcriptional regulation - to control amount of cyclins
- phosphorylation/dephosphorlyation of Cdks
- Cdk kinase inhibitors - in the control of G1/S- and S-Cdk
- ubiquitin-dependent proteolysis -to control amounts of cyclins (in addition to transcriptional control)
Provide an example of the phosphorylation/dephosphorylation of Cdks to regulate their activity.
Cdk is first activated by phosphorylation at its active site, regardless of the presence of cycln
M-cdk:
it can be phosphorylated at a second site by Wee1 kinase, inhibiting it
this phosphate can be removed by cdc25 phosphatase, reinstating the activity of cdk

Provide an example of a Cdk inhibitor that controls activity of Cdk.
In G1/S- and S-Cdks, p27 may bind the G1/S-cdk and G1/S cyclin complex, restricting access to the active site.

Describe ubiquitin-depedent proteolysis to control Cdk activity by APC/C.
In the case of M-Cdk:
Cyclins can be degraded by ubiquitination by APC/C when it is in its active state. APC/C is normally inactivated by Cdc20

Describe ubiquitin-depedent proteolysis to control Cdk activity by SCF.
promotes S-phase entry:
- an active SCF complex contains an F box protein with catalytic activity
- F box ubiquitinates CKI protein when it is active and phosphorylated, allowing for the G1-S transition.

How do S-Cdks license the cell to only replicate DNA once per cycle?
Cdc6 and Cdt1 are licensing factors involved in forming the pre-replicative complex at the ORC. At this point, S-Cdk is active and causes origin firing. It also phosphorylates cdc6 and cdt1, marking them for degradation so that another replicative complex cannot be formed.

When and how is sister chromatid cohesion established?
Sister chromatid cohesion is established in S phase, after DNA has been replicated.
- In M1-S, cohesin is loaded around DNA molecule.
- In S, cohesion is established as cohesin proteins line up at replication forks. The two daughter molecules pass through rings of cohesin.
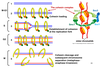
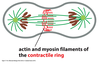
List the stages of mitosis.
- prophase
- prometaphase
- metaphase
- anaphase
- telophase
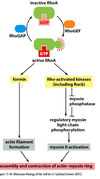
Describe the G2-M transition and how M-Cdk drives entry into mitosis.
Once Cdk1 is bound by cyclin and activated by Cdk-activating kinase, it is often inhibited by Wee1 kinase. Cdc25 will come along and remove this inhibitory phosphate, reactivating Cdk1. Activation of Cdk1 acts as a positive feedback loop, as it inhibits wee1 kinase and activates cdc25 phosphatase, leading to the accumulation of Cdk1 after the initial activation of one molecule.

Describe in detail the condensation of DNA. When does this take place?
DNA is condensed into chromosomes during prophase of the M phase.
- condensin catalyzes the restructuring and compaction of chromosomal DNA.
- condensin is similar in structure to cohesin. It contains the Smc proteins organized into a ring structure, with 3 non-SMC proteins.
- by metaphase, we can see siste chromatid resolution

Describe nuclear envelope breakdown. During what phase of mitosis does this occur?
Nuclear envelope breakdown occurs during prophase, prometaphase, and metaphase. It is facilitated by M-Cdk
- M-cdk phosphorylates some proteins in nuclear pore complexes, the nuclear lamins, and inner nuclear envelope proteins
- this causes disassembly of nuclear-pore complexes and the breakdown of nuclear membranes into small vesicles
- this leads to the release of motor proteins and microtubule regulators from the nucleus to the cytoplasm for mitotic spindle formation
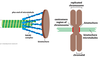
Describe mitotic spindle formation. When during mitosis does this take place?
Mitotic spindle formation takes place during prophase, prometaphase, and metaphase of mitosis.
- astral microtubules contact the cell cortex
- kinetochore microtubules attach to sister chromatids via their kinetochores.
- interpolar microtubules overlap with the plus ends of microtubules from the opposite pole to form an antiparallel array at the midzone

Where are the mitotic spindle poles located? How do these structures duplicate?
They are assembled at opposing centrosomes. These are made up of two centrioles each, surrounded by a pericentriolar matrix consisting of:
- microtubule-dependent motor protiens
- gamma tubulin (responsible for nucleating microtubules)
Centrosome duplication is promoted by G1/S-Cdk. The two centriole pairs remain close to one another until ready for mitotic spindle formation. Centrosomes replicate in a semiconservative manner, just like DNA.

How is the assembly and function of the bipolar mitotic spindle governed?
It is governed by Microtubule-dependent motor protiens:
- kinesins move towards plus ends
- dyneins move towards minus ends (help position mitotic spindle in cell via astral microtubules)
- they push and pull microtubules to separate the spindle poles

How are motor proteins which govern mitotic spindle formation activated?
Kinesin and dynein are activated by phosphorylation by M-Cdk,polo-like kinase, and aurora kinases A and B.
How do kinetochores attach sister chromatids to the mitotic spindle?
- spindle microtubules attach to the outer region of large kinetochore complex
- kinetochore complex forms at centromere region of chromosome
- plus ends of microtubules are embedded in the fibrous part of the kinetochore that contains Ndc80.
- Ndc80 attaches to the sides of microtubules, so they can continue to polymerize and depolymerize.