CF\LF Theory - Mixed Questions Flashcards
Deck imported from "Chem 402 Inorganic Chemistry". Original deck name: "Ligand Field Theory". (27 cards)
Which orbitals are non-bonding in a Td symmetric crystal field?
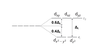
e

Which represents an orbital set that would have e symmetry in a Td crystal field?

D
Tetrahedral transition metal complexes are always_____spin.
High or Low spin
High Spin
In an Oh crystal field, the eg orbitals are destabilized.
What is the value of A?

6
In an Oh crystal field, the t2g orbitals are stabilized.
What is the value of B?

-4
Mixing ligand π charater (π-donor) into the t2g set of orbitals in an Oh ligand field results in the t2g set becoming more _________.
Bonding or Antibonding
Antibonding
(Ligand π orbitals are lower in energy than the t2g set and therefore any mixing results in HOMO frontier orbitals being of antibonding character)

Td=AΔo
What is the value of A?
4/9
(There are only 4 ligands in a tetrhedral complex. Therefore, the ligand field is roughly 2/3 of the octahedral field)
What is the splitting energy of the d-orbitals in an octehedral crystal field?
10Dq or Δo

What is Δo in terms of Dq?
10Dq
What is the symmetry of the orbital set below in a Oh ligand field?
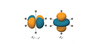
eg
What is the symmetry of the orbital set below in a Oh ligand field?

t2g
As the principal quantum number increases, ∆o_______.
Increases or Decreases
Increases
(Larger orbitals result in stronger interaction with ligands)
As the oxidation state increases,∆o_______.
Increases or Decreases
Increases
π-Acceptors_____∆oh
Increases or Decrease
Increase
π-Donors_____∆oh
Increase or Decrease
Decrease
CO, H-, PPh3
Strong or Weak field ligands?
{Rank them}
Strong Field Ligands
PPh3<h>-<co></co></h>
Total Spin
Stot= (# unpaired e-)/2
2*(Stot)+1
Spin Multiplicity
Quintet
Stot=2
Triplet
Stot=1
Doublet
Stot=1/2
Singlet
Stot=0
I- ,H2O ,Cl-
Strong or Weak field ligands?
{Rank Each}
Weak Field Ligands
I- < Cl- < h2O
Which represents an orbital set that would have t2 symmetry in a Td crystal field?

C





