chapter 15 Flashcards
(10 cards)
1
Q
what is eqilibrium?
A
- there is no net change in reactant and product concentrations over time
- the rates of the forward and reverse reactions are equal

2
Q
what does the equilibrium constant depend on?
A
temperature
- increasing temperature results in increased Kc
3
Q
what is the law of mass action?
A
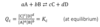
4
Q
when is it helpful to calculate Qc?
A
when the system is not at equilibrium

5
Q
what is significant about the concentrations of pure liquids and solids?
A
concentrations of pure liquids and solids are constant values, so they can be omitted from the equilibrium constant expression
6
Q
how is pressure and concentration related?
A

7
Q
how is KP denoted?
A

8
Q
relationship between Q and K
A
- Q < K : reactants must be converted to products b/c there is a shortage of products
- Q = K : equilibrium
- Q > K : products must be converted to reactants b/c there is a shortage of reactants
9
Q
changes in volume and pressure and its impact on equilibrium
A
- decrease in volume = decrease in the total number of moles of gas
- increase in volume = increase in the total number of moles of gas
10
Q
changes in temperature and its impact on equilibrium
A
- increase in temperature = endothermic reactions (+) will go forward and exothermic reactions (-) will go back
- decrease in temperature = endothermic reactions (+) will go back and exothermic reactions (-) will go forward
- temperature increase favors an endothermic reaction and a temperature decrease favors an exothermic reaction


