Chapter 2 Chemistry Flashcards
(120 cards)
What does chemistry have to do with physiology?
- Your entire body is made up of chemicals
- Chemical reactions underline all processes that occur in the body
Your entire body is made up of what?
Chemicals
What underlines all the processes that occur in the body?
Chemical reactions
what is an example of processes in the body that are caused by chemical reactions?
- Digestion
- Muscle movement
- your heart pumping blood
What kind of reaction is cellular respiration?
Redox (reduction-oxidation)
What is the chemical equation for cellular respiration?
C6H12O6 + 6O2 ———> 6CO2 + 6H2O + Energy + Heat
Glucose + Oxygen = Carbon dioxide + Water + ATP + Heat
What are the types of chemical bonds?
- Covalent Bonds
- Hydrogen Bonds
- Ionic Bonds
Covalent Bond
A bond formed by atoms sharing electrons
- do not conduct electricity
- do not easily dissolve in water
- tend to be gases or softer solids
Electronegativity
The ability of an atom to attract shared electrons. Oxygen is electronegative (electron greedy). Electrons spend more time around the more electronegative atom. In the case of hydrogen and oxygenthe electrons spend more time in closer proximity to oxygen.
Polarity
The separation of charges.
ex. a polar covalent bond
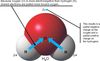
polar covalent bond
A covalent bond in which the two atoms have different electronegativities, causing a separation of charges.
Nonpolar Covalent bond
a covalent bond in which the two atoms have identical or very similar electronegativities so that the charges are distributed evenly.
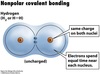
Covalent bonds are usually formed by what?
Nonmetals and metalloids
Electron shells of metals…
metals have loosley held outer electron shells, they consistantly drop electrons and become positive ions.
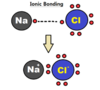
What creates an ionic bond?
When positive ions meet negative ions such as those found in the hallogen family, they are attracted to eachother, they bond to keep their energy use at the lowest possible minimum.
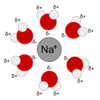
Ionic bond
A bond formed by the transfer of electronsfrom one atom to another.
- They are extremely polar; more than polar covalent bonds
- ions usually have a crystaline form
- usually dissolve in water
- ions once dissolved in water give the water the ability to conduct electricity.
Hydrogen Bonds
The attractive force between the hydrogen attached to an electronegative atom of one molecule and an electronegative atom of another molecule.

- Attraction between a positive and a negative
What are human made out of?
matter
Matter
anything that occupies space and has mass
What are the states of matter?
- Liquid
- Solid
- Gas
Energy
the capacity to do work
What are the types of energy?
- Kinetic
- Potential
Kinetic Energy
energy in motion