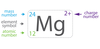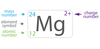Chapter 4 Flashcards
(58 cards)
4-1
An element, when we mean a single atom of that element it is called the _____ form of an element.

Microscopic

The term element, we mean a sample of the element large enough to weigh on a balance. It is called the _____ form of an element,

Macroscopic

4-1
Ex: The human body contains the element sodium or lithium (arsinic). This is a form of an element in a _____.

generic fashion

4-2
The abbrivation terms given to chemical elements are called the _____.

element symbols
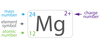
4-2
The term for essintial chemical compounds and elements found within the body are _____.

Trace Elements

4-3
The _____ ______ _____ _____ states that a given compound always contains elements in exactly the same proportion by mass.

law of constant composition

4-3
This theory is called _____ _____ _____. It states that:
- Elements are made of tiny particles called atoms.
- All atoms of a given element are identical.
- The atoms of a given element are different from those of any other element.
- Atoms of one element can combine with atoms of other elements to form compounds. A given compound always has the same relative numbers and types of atoms.
- Atoms are indivisible in chemical processes. That is, atoms are not created or destroyed in chemical reactions. A chemical reaction simply changes the way the atoms are grouped together.

Dalton’s Atomic Theory

4-4
A _____ is a substance with constant composition that can be broken down into elements by chemical processes.

compound

4-4
In Dalton’s Atomic Theory, in the context that water always contains two hydrogen atoms for each oxygen atom, the term relative refers to _____.

ratios

4-4
A _____ is:
A representation of a molecule in which the symbols for the elements are used to indicate the types of atoms present and subscripts are used to show the relative number of atoms

chemical formula

4-4
In a chemical formula, the atoms are indicated by the ______ symbols.

element
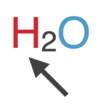
4-4
In the chemical formula, the number of each type of atom is represented by the ______.

Subscript

4-4
The types of atoms and the number of each type in each unit (molecule) of a given compound are conveniently expressed by a _____.
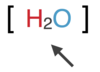
chemical formula

Just try to remember this one.

Yup - Need to work on this one.

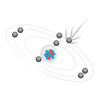
4-5
In this ataom model, what is number 1 called?

electron

4-5
In this atom model, what is number 2 called?

nucleus

4-5
In this atom model, what is number 3 called?

neutron
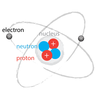
4-5
In this atom model, what is number 4 called?

proton

4-5
A/an _____ is a type of particle. It is found within the space around the nucleus of an atom. It is negativly charged.
electron

4-5
The _____ is the center of an atom. It is made up of nucleons (protons and neutrons) and is surrounded by the electron cloud.

nucleus
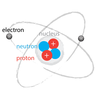
A _____ is a type of particle. It is found in an automic nucleas. It is positivly charged.

Proton

4-5
_____ _____ consist of two protons and two neutrons bound together into a particle identical to a helium nucleus.

alpha particles

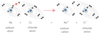
4-6
_____ are:
Any of two or more forms of a chemical element, having the samenumber of protons in the nucleus, or the same atomic number, buthaving different numbers of neutrons in the nucleus, or differentatomic weight.

isotopes

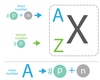
What is the Isotope Notation?

View image.