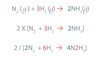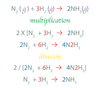Chapter 6 Flashcards
(32 cards)
A _____ reaction is when substances are changed into other substances.

chemical

_____ are compounds and or elements.

Substances

In a chemical reaction, there are a number of _____ that show that a chemical reaction has taken place.
They include:
- Visibly
- Heat is given or taken in

clues
(Urine Analysis DipStick, Macro)

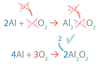
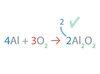
In an equation, ______ makes sure that all atoms present in the reactants are accounted for among the products
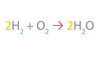
balancing
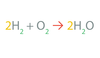
These are _____.

Reactants

These are _____.
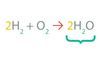
Products

These are _____.

Arrow

These are _____.

Coefficients
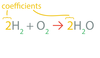
A chemical equation shows the _____ and _____.

(And other factors)
reactants and products

Reactants are chemical substances that are eventually _____.
It is number _____.

changed
4

Products are chemical substances that are _____.
It is number _____.
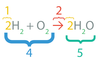
formed
5

The arrow in a chemical formula shows that a chemical reaction _____ taken place.
It is number _____.

has
2
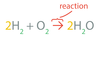
A Chemical Reaction’s Formula Format is as follows:
_____ ➞ _____.
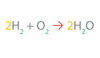
Reactants → Products

The Coefficients indicate the number of each _____ species that reacts or is formed.
It is number _____.

chemical
1

the (aq) symbol stands for _____ _____ _____.
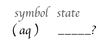
in aqueous solution.

the (g) symbol stands for _____ _____ _____.
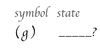
gass

the (l) symbol stands for _____ _____ _____.

liquid

the (s) symbol stands for _____ _____ _____.
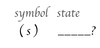
solid

The law of conservation of mass states that matter is neither _____ nor ____.

created nor destroyed

Chemical Equations Obey the law of _____ of _____.
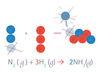
conservation of mass.

Chemical Equation Calculations are used to calculate how much of each _____ is needed and what will be _____.

element
produced

A Balanced Equation has the same number of each _____ of _____ on both sides.

kind of element

Within Chemical Equations (Compounds) – The compounds or the subscripts (can or can not) be changed. This will not balance the equation. Because that would change the compound.
can not
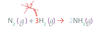
Chemical Equations (Coefficients) – The coefficients (can or can not) be placed in front of compounds or elements in the equations. This will balance the equation.

can