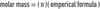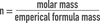Chapter 8 Flashcards
afwe (65 cards)
In the mass/molar/atoms conversion,
if (mass==amu), then what formula is used to solve?

{atoms = 1 atoms}

In the mass/molar/atoms conversion,
if (mass==grams), then what formula is used to solve?

{atoms = 1 Na atoms / mass}

In the mass/molar/atoms conversion,
if (mass==moles), then what formula is used to solve?

{moles = 1 Na atoms / 1 mole}

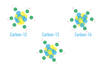
The _____ _____ mass of an element is massed average of masses of all isotopes.
average atomic mass
The _____ mass of a substance is the mass of 1 mole of the substance. (in grams)

molar mass
The _____ _____ of an element present in a compound can be obtained by comparing the mass of the particular element present in 1 mole of the compound to the molar mass of the compound.
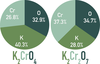
mass fraction
The mass fraction of an element present in a compound can be obtained by comparing the mass of the particular element present in 1 mole of the compound to the _____ mass of the compound.

molar mass
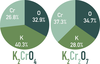
To determine the _____ formula of a new compound, the composition of the compound by mass must be known.

empirical formula
To determine the _____ formula of the compound, the molar mass of the compound must also be known.

molecular formula
(Composition of the compound by mass and molar mass of the compound must be known before its molecular formula can be determined.)
In unit analysis, you are essentially multiplying by a ratio that equals “______“.

one
Avogadro’s number = _____ <span>E</span> _____

_6.022_E23
(or 6.022x1023)

The _____ _____ mass of an element is equal to the sum of masses of it’s isotopes, each multiplied by it’s natural abundance.

average atomic mass
The _____ _____ Unit (or _____) is 1/12 the mass of an atom of carbon 12.
In other terms, it is the average of the proton rest mass and the neutron rest mass. Approximately 1 ____ = _____ g.

Atomic Mass Unit (or AMU)
1 AMU = 1.66E-24 g
( in grams)
In samples where the ratio of the masses is the same as the ratio of the masses of individual atoms, they always contain the same number of _____.

atoms
In a sample where all elements are weighed, each sample has a ____\_ equal to that element’s average atomic mass in grams. These samples all contain the same number of atoms.

mass
The _____ (or _____) is a number of carbon atoms in exactly 12 grams of pure 12C

mole (or mol),
The _____ number is one mole that represents 6.022E23 units.

Avogadro
The _____ (or _____) is a number equal to the number of carbon atoms in 12.01 g. of carbon.
(in grams)

mole (mol)
(This is slightly different than SI unit’s standards.)
In any sample, one mole of anything is ______ units of that substance.

6.022E23
In these samples of pure elements, they contain the same number of ____\_ or 1 _____.

atoms or 1 mole
In a sample of pure graphite (a form of carbon),
it weights _____ g.

12.01 g
(why is this important? who knows!)
In a sample of an element with a mass equal to that element’s average atomic mass expressed in grams contains ___ _____ of atoms.

1 mole
A _____ _____ is fundamentally a collection of atoms.

chemical compound
The _____ mass is the mass in grams of mole of a compound

molar mass