Chemistry Flashcards
(93 cards)
What are the pH of acids?
Acids = 0 - 6
What are the pH of bases?
Bases = 8 - 14
Are metal hydroxides acids or alkalis/bases?
Bases
Are metal carbonates acids or alkalis/bases?
Alkali/bases
Are metal oxides acids or alkalis/bases?
Alkali/bases
What is the difference between an alkali a base
Bases are solid, alkalis are soluable bases
Define amphoteric
Can act as an acid and a base
What is a general equation for a neutralisation reaction?
Acid + Alkali –> Salt + Water
Write balanced symbol equations for the neutralisation reactions between hydrochloric acid and sodium hydroxide.
HCl + NaOH –> H₂O + NaCl
Acid is a ___ donor
Proton (H+)
Alkalis produce what ion in solution
OH-
Alkali is a ___ acceptor
H+
Acid creates ___ in water
H+
Bases ______ an acid
Neutralises
All alkalis are ___
Bases
What is a nucleophile?
An electron rich species, e.g. a species with a long pair or a negative charge
What is an electrophile?
An electron poor species, e.g. positively charged species
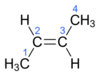
Outline the mechanism for the electrophillic addition of HBr to ethene

State uses for alumina (Al2O3)
The major uses of aluminium oxide is in refractories, ceramics, polishing and abrasive applications.
Describe what happens to ions (Al3+ and O2-) when Al2O3 is electrolysed
Al3+ gains electrons and forms pure Aluminium (Reduction), O2- looses electrons and forms Oxygen gas (Oxidation)
State half equations for the reactions at the electrodes
Anode) 2O2- —> O2 + 4e- Cathode) Al3+ + 3e- —> Al
Describe the reactions at the electrodes as oxidation/reduction
The reaction at the anode is Oxidation (losing electrons), and the reaction at the cathode is Reduction (gaining electrons)
Explain why electrolysis of alumina is expensive
It’s expensive due to the large amount of electricity required to keep the alumina (bauxite) molten to allow the electrolysis to continue 24/7, 365
State uses of titanium
Titanium metal is used as an alloying agent with other metals (e.g. aluminium). Alloys of titanium are used in aerospace, aircraft and engines due to the strong, lightweight, temperature-resistant properties.