Lec 5- Nucleic acid based systems Flashcards
(46 cards)
1
Q
The central dogma
A
- Target early in this pathway and you can take advantage of the amplification mechanism

2
Q
Introduction to gene therapy
A
- Gene therapy is the insertion, alteration or removal of genes within individual cells and biological tissues to treat diseases caused by genetic disorders
- A number of human diseases are known to be genetic in origin e.g. CF, Huntington’s and cancer
- The therapeutic genes: DNA, oligonucleotides, siRNA and mRNA
3
Q
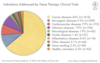
Indications addressed by gene therapy clinical trials
A
- Most diseases treat via this pathway is cancer- genetic factor, lots of funding
- Monogenic disease- single change in a gene leading to a disease- far easier to target than other diseases
4
Q
History of gene therapy
A
- 1998, Fomivirsen was the first antisense oligonucleotide approved by the FDA. It was used in the treatment of CMV in immunocompromised patients
- In 2003, the Chinese FDA approved the controversial and first adenovirus-based gene therapy for head and neck squamous cell carcinoma
- 2004, Pegaptanib anti-VEGF aptamer, was marketed in the USA for age-related macular degeneration
5
Q
From Lab to the clinic: progress so far
A
- NB- Large failure rate

6
Q
Mechanism of gene therapy
A
- Potency potential of gene therapy

7
Q
Cell transfection
A
- Genetic material enters into the cell- (Similar to virus)
- We will then interupt the centeral dogma- either put correct gene in or knock out deffective gene
- In the endosome the DNA is not technically in the cell yet so we need to be able to release it
- Then cross the nuclear membrane
- Transcrption of functional protein

8
Q
Types of gene therapy
A
- Bind to genomic DNA in the nucleus and thus block transcription
- The guide strand of siRNA activates the RNA-induced silencing complex (RISC) and then degrade the mRNA (RNA silence)
- A complimentary (antisense) oligonucleotide bind their target (sense) mRNA and block the translation
- Plasmid= insert a functional gene/ missing gene (Autoimmune disease)- must be in the nucleus
- Antisense oligonucleotides/siRNA- block gene

9
Q
Example 1: Pegaptanib
A
- The first aptamer to be successfully developed as a therapeutic agent in humans- a milestone in drug development
- In 2004, FDA approved pegaptanib an Anti-Vascular Endothelial Growth Factor (anti-VEGF), RNA aptamer
- The treatment of all types of age-related macular degeneration

10
Q
Biological barriers to gene delivery
A
- Once in the endosome, we need to ensure it stays stable
- And we need to ensure that we can get it out of the endosome to enter to the cytoplasm or transfer into the nucleus

11
Q
A

12
Q
Potential disadvantages and problems
A
- Short-lived nature of gene therapy: susceptible to degradation
- Trigger immune response- put DNA into a cell and recognises the new DNA (it shouldn’t be there, the immune cells will destroy the cells- do more damage)
- Multigene disorders: high blood pressure, Alzheimer’s disease, arthritis and diabetes
- More than one genetic factor can complicate things
- Polyanions
- Surfaces of cells and DNA are both negative therefore is a difficult membrane to cross
13
Q
Summary of oligo action
A
- Site of action
- Genomic DNA- active site= nucleus
- mRNA- active site= cytoplasm
- Protein- active site= cytoplasm

14
Q
Chemical modification of nucleic acids
A
- Unmodified plasmid DNA, siRNA and phosphodiester (PO) backbone oligos are rapidly degraded by enzymes in biological fluids
- To overcome the instability of oligos, chemically modified oligos have been developed

15
Q
Phosphodiester backbone
A
- Phosphorothioate oligos
- Non-Bridging oxygen is replaced with sulphur
- Swap out unbound oxygen with sulphur (Disulphide bond more stable)- less sensitive to degradation so is stable invivo and hydrophobic= partition more into the membrane

16
Q
Example 2: Fomivirsen
A
- The first antisense oligomer approved by the FDA in Aug 1998
- A synthetic 21 member oligonucleotide with phosphorothioate linkages (which are resistant to degradation by nucleases
- Sequence: 5’-GCGTTTGCTCTTCTTCTTGCG-3’
- Treatment CMV in immunocompromised patients, including those with AIDS
- Mechanism: Bind to the complementary sequence of the mRNA to block translation of viral mRNA
- Administrated by intraocular injection
17
Q
Example 3: Mipomersen
A
- It is a cholesterol-reducing drug candidate
- It targets the messenger RNA for apolipoprotein B
- A second-generation antisense oligonucleotide
- The nucleotides are linked with phosphorothioate linkages
- The sugar parts are deoxyribose in the middle part of the molecule and 2’-O-methoxyethyl-modified ribose at the two ends
- combination of RNA and DNA- the steric arrangement is different therefore the change of shape = won’t fit inactive site- change function
- These modifications make the drug-resistant to nucleases

18
Q
Modification of sugar
A
- Losing the hydrophobicity may actually be benefitial

19
Q
Locked Nucleic acids (LNA)
A
- The ribose ring is locked by a methylene bridge connecting the 2’-O atom and the 4’-C atom- strengthening the overall molecule
- Discovery in 1997 by Danish scientists in Danish
- The locked ribose conformation enhances base stacking and backbone pre-organisation
- More stable
- Increase the sensitivity and specificity

20
Q
Peptide Nucleic Acid (PNA)
A
- PNA: Consisting of repeating N-(2-aminoethyl) glycine units linked by peptide bond, the bases are attached to the backbone through methylene carbonyl linkages
- PNA is being produced to match DNA

21
Q
PNA features
A
- The stronger binding between PNA/DNA strands then between DNA/DNA strands
- H-TGTACGTCACAACTA-NH2 , It’s Tm is 69.5 ‘C - more stable
- The corresponding DNA-DNA duplex Tm is 53.3 ‘C
- 1’C higher per base pair on average
- A mismatch is a PNA/DNA duplex usually causes more destabilisation than a mismatch in DNA/DNA duplex
- E.g. for a 15 per PNA, it’s average deltaTm is 15’C, whereas average deltaTm for the corresponding DNA/DNA duplex is 11’C
- Significantly higher rate of hybridisation in assays where either the target or the probe is immobilised
- Stable to nucleases and proteases as it is neither a DNA nor a peptide
- Recent research in our lab found out that an 18-mer antisense PNA inhibited BCL-2 protein with liposome as the delivery vehicle
22
Q
Melting point of DNA duplex
A
Don’t worry about this slide

23
Q
Modification of heterocycles
A
- Very similar structure
- Changes can change stability

24
Q
Nucleic acid delivery systems
Vectors for gene delivery
A
- Physical methods for gene delivery microinjection, gene gun
- => Disadvantage- practicability problem in the human body
- Viral vectors, Adenoviruses, retroviruses, HSV
- => Inherent drawbacks- Strong immune response, Risk of oncogenesis
- Non-viral vectors lipid, PLL, PEI, PAMAM
- Limitations- Low transfection efficiency, High toxicity
25
Vectors used in nucleic acid delivery systems

26
Physical methods in gene delivery
* A) **Electroporation:** Uses short pulses of high voltage of electric current to carry DNA across the cell membrane
* This shock causes the temporary formation of pores in the cell membrane, allowing DNA molecules to pass through
* B) **Gene gun:** DNA is coated with gold particles and loaded into a device which generates a force to achieve penetration of DNA/gold into the cells
27
Physical methods in gene delivery
* C) **Sonoporation:** Uses ultrasonic frequencies to deliver DNA into cells
* The process of acoustic cavitation is thought to disrupt the cell membrane and allow DNA to enter into cells
* D) **Magnetofection:** DNA is complexed to magnetic particles, and a magnet is placed underneath the tissue culture dish to bring DNA complexes into contact with a cell monolayer
* Pulling nanoparticles into the cells with the magnets
28
Viral-vectors in gene delivery
* A virus is a small infectious agent that can replicate only inside the living cells of organisms
* Viruses have a natural ability to infect cells
* Virus particles (virions)
* Genetic material made from either DNA or RNA
* Protein coat that protects these genes
* Lipids that surrounds the protein coat
29
Pathway of viral infection
* Influenza virus becomes attached to a target epithelial cell
* The cell engulfs the virus by endocytosis
* Viral contents are released. Viral RNA enters the nucleus where it is replicated by the viral RNA polymerase
* Viral mRNA is used to make viral proteins
* New viral particles are made and released into the extracellular fluid. The cell, which is not killed in the process, continues to make new viruses

30
Viral-vectors in gene delivery
* Involves the use of attenuated or defective viruses
* A) A retrovirus (An RNA virus)
* X-linked severe combined immunodeficiency (X-SCID)- the most successful application of gene therapy to date
* Problem:
* Insert the genetic material into any arbitrary position in the genome of the host (Not targeted). Five children in the trial have developed leukaemia as a result of insertional mutagenesis by the retroviral vector
31
Viral-vectors in gene delivery
B)
* B) Adenovirus (DNA virus)
* Gendicine, adenoviral p53-based gene therapy was approved by the Chinese FDA in 2003 for head and neck cancer- very safe, decreased efficacy
* Advexin, a similar gene therapy approach from Introgen, but turned down by USFDA in 2008
* Death of Jesse Gelsinger in 1999 while participating in a gene therapy trial
32
Viral-vectors in Gene Delivery
C+D)
* Envelope protein pseudotyping of viral vectors
* Herpes simplex virus (HSV)
33
Glybera
* The EMA approved Glybera
* For the treatment of lipoprotein lipase deficiency
* LPLD: A very rare inherited condition that is associated with increased levels of fat in the blood
* Glybera introduces a normal, healthy LPL gene into the body so that it can make functional LPL protein
* It consists of the LPL gene packaged in a non-replicating adeno-associated virus (AAV) which has a particular affinity for muscle cells- which is its active site
* It is administered via one-tome series of up to 60 small intramuscular injections in the legs
34
Non-viral vectors in gene delivery
* Cationic- charges won't repel each other
* Steric stabilisation- prevent degradation
* targeting ligands- look at what ligands (including proteins) make up the membrane

35
Masking of anionic charges
Advantages of masking anionic charges of nucleic acids by cationic delivery systems
* Condenses the size of the nucleic structure
* Helps protect from nuclease degradation- smaller less likely to interact as well as different shape for nuclease enzymes
* These enzymes bind to negatively charged nucleic acid systems
* Help improve circulation time
* Avoids recognition by the kupffer cells
* Improve cellular uptake
* This is the end goal/main focus- all of these process are improve to increase uptake
36
Cationic Liposomes
| (Masking of anionic charges)
* Due to the anionic nature of the nucleic acids, cationic liposomes are used to electrostatically bind to the nucleic acids
* Lots of NH2 groups
* This can
* Condense DNA (reduce the size)
* Mask its anionic nature
* Protect it from nuclease degradation
* Very well packaged- no external interactions, less chance of breaking

37
Basic components of a cationic lipid
1. A hydrophobic lipid anchor group which helps in forming liposomes and can interact with cell membranes
2. A linker group
3. A positively charged head group
1. DOTMA
2. DOTAP
3. DC-ChE
4. Often co-lipid DOPE is included in formulations
* These charged head groups, give a similar structure to standard phospholipid layer
* This means interactions between surface of liposomes and cells that it approaches

38
Dioleolyl phospatidylethanolamine (DOPE)
* Co-lipid commonly used in liposomes used for nucleic acid delivery
* Though to facilitate the release pDNA (plasmid DNA) from endosome (Efficient release of the pDNA from the endosomal compartment is a limiting step in gene expression)
* When DOPE-containing liposomal-DNA complexes are taken up by the endosome, all of the cationic lipid headgroups are neutralised by the anionic lipids in the endosomal membrane
* Promote membrane breakdown in acidic conditions (e.g. endosomes)

39
Membrane fusion theory- Endosome escape
* Step A: Cationic liposome/nucleic acid complex is endocytosed
* Step B: In the early endosome, membrane destabilisation results in anionic phospholipid flip-flop
* Step C: The anionic lipids diffuse into the complex and form charge-neutral ion-pairs with the cationic lipids
* Step D: The nucleic acid dissociates from the complex and is released into the cytoplasm

40
Flip flop
* Red= part of original cell membrane
* membranes fuse together

41
Cationic liposomes: Delivery objectives
* Correctly target required site
* Use targeting groups on the surface of liposomes, e.g. Ab's
* Protect against degradation
* Neutralise charge and protect with a lipid coat
* Enhanced cellular uptake
* Improve stability and improve the amount of reaching cells
* Cell uptake thought to be via endocytosis (Improve using receptor-mediated endocytosis)- DOPE, flip flop
* Improved exit from sub-cellular compartments
* Incorporate DOPE in the formulation which improves release via flip-flop mechanism
* Improve entry into the nucleus if required for action
* Free DNA released from endosome may enter the nucleus through pores in the nuclear membrane
42
Polymer-based gene delivery system
* Protonated amine group (NH3+) interact with phosphate groups of the NA's
* This can condensing DNA, reduce the size, mask its anionic nature and protect it from nuclease degradation
* Vary in terms of their molecular mass, their shape
* Their backbone can also be modified by the introduction of side chains or target-specific moieties
43
Proton sponge hypothesis- endosome escape
* The cationic polymer (such as PEI) soaks up the H+ ions and becomes more cationic at low pH, leading to inflow H+ and Cl- and water into the endosome
* This causes osmotic swelling, burst open the endosome and releasing the DNA
44
Cationic polymers: PEI
* Composed of: Primary amine; Secondary amines; **Tertiary amines**
* **Lots of hydrogen**
* This increase in protonation as the pH drops can have a buffering effect inside the endosome
45
Bio-reducible disulphide-linked polymer
* Disulphide- linked polymers are not stable intracellular due to high glutathione concentrations, but stable extracellular due to low glutathione concentrations
* Positive charge on the surface= better uptake into the cell

46
Disadvantages of non-viral systems
* Very low transfection efficiency
* Rapidly cleared by the reticuloendothelial system (RES)
* After IV injection high expression in the lungs has been noted
* Clearance may be reduced by pegylation
* Local injection of complexes have shown to be ineffective


