Lecture 3 Flashcards
(34 cards)
Basic strucutre of a neuron
- Cell body Dendrites Axon

Cell body
- Cell body houses the nucleus and other typical cell organelles
- plasma membrane around the cell body is characterized by local potentials
- Voltage-gated ion channels are not characteristic of the cell body membrane
Dendrites
- Dendrites are cellular extensions of the neuron
- Although variable, the number of dendrites is typically a few to many
- dendrites are characterized by the presence of ligand(neurotransmitter)-gated ion channels
- Dendrites conduct local potentials
Axon
- neuron charecterized by a single axon that is variable in length
- Axon is an etension of the cell body and is typically opposite the side of the cell body where the dendrites are located
- Distal end of the axon is cahrecterized by the presence of membrane bound vesicles filled with neurotransmitter molecules
Axolemma
- Characterized by the presence of voltage -gated ion channels and the ability to conduct an action potential
- The axon is an extension of the cell and is covered by the plasma membrane (referred to as the axolemma)
Concentration gradient
- Cell membrane or plasmalemma,functions to maintain separate intracellular and extracellular environmnts
- Difference in ion concentrations(dichotomous distribution of ions) can change depending on whether or not the plasmalemma is permeable to specific ion at a given periods of time
What are the concentration of ions inside and outside the cell?
- Concentration of sodium and chloride ions are much more highly concentrated outside the cell
- potasium ions are considerably more concentrated inside the cell
- Diffusion Potential
Diffusion potential is caused by an ion concentration difference on either side of a membrane
Nernst potential
- Nernst potential is the idffusion potential level across a membrane that exactly opposes the net diffusion of a particular ion through the membrane
Nernst equation
E=2.3RT/F logCo/Ci
- E=difference in the electrical potential between inside and outside the neuron=Nernst potential
- R=niversal gas constant
- T=absolute temperature
- F=electric charge per gram equivalent of univalent ions
Used to determine the diffusion potential across a membrane that exactly opposes the net diffusion of a particular ion through the membrane. It measures the potential for one type of ion at a time
To measure the combined potential for more than one type of ion, the goldman equation may be used
Assumptions:
- Equation can only be used for one ion at a time
- Membrane must be completely permeable to that ion
- Ion must be at equilibrium
Electrial Dipole Layers

- Positive and negtaive charges are distributed somewhat evenly within both the extracellular and intracellular fluids.When both the indifferent electrode and the rerdoign electrode are in the same fluid, the voltage will read as “0”
- However ,the distribution of ions immediately on either side of the membrane is mostly positive or mostly negative. This dichotomous distribution of ions on either side of the membrane represents a voltage change area called the elctrical dipole layer
- The potential electrical difference across the cell membrane is recorded as negative, because the recording electrode is inisde the membrane
a. Recording electrode is in the extracellular fluid
B.Recording electrode peirces cell membrane
C.Recording electrode pierces other side of membrane and is in the extracellular fluid again
Simplified Nernst Equation
- By combinign the constnats ,converting to log base 10 instead of natural log, and considering the potential at body temperature , the above equation can be simpliefied to:
- Eion=z(61.5)xlog([ion]outside/[ion]inside)
- z=valence of ion , and nomrally valence is 1 unless doing with calcium(and have to be concerned with if its negatvie or positive)
Diffusion Potential

- Going to reach an equilbirium where ions are equal on both side and our voltage shoud be zero as we reach zero
- Sodium ions drag larger clouds of water molecules that chloride ions,so chlordie is more mobile
- The left side will be negative until concentration equilibrium is reached
- Describes a transient situation
- This scenario does not describe an equilibrium condition but rather transient situation that exists as long as there is net diffusion
Equilibrium potential

- Equilbiruim will be reached when the electrical force driving chloride ions out of hte left hand compoartment exactly balances the concentraional force driving chloride ions out of hte right hand compartment
- Equilibrium for an ion is determined not only by concentraional forces but also by electrical forces
- Chlordie is more concentrated on outside then insdie , and expecte it to move insdie but as we accumulate more negative charges , then some are going to repel and stay on the outside
- two forces working here , electrical and chemical
- Use Nernst equation to calcualte electromotive force for chloride ion
- -61.5mV
Principle of electrical Neutrality
What are the concentrations of the ions indicated by the question marks?
use the nernst equation to calculate the elctromotive force for the chloride ion
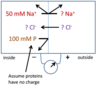
- Under biological conditions the sum of the concentrations of cations within any compartment must be equal to the sum of the concentrations of anions in that compartment.
- in this model both osmotic and electrical factors must be considered in equilibrium
- Begin on left side, balance the negative with the positive so chloride would need to be 50 total concentraion will be 200
- Cl- will be 100 on left side and 100m for Na+
- Electrive motive force would be -18.4mV
Donnan(Gibbs-Donnan)Equilibrium

- If equilibrium is to be reached with two permeant ions(ex. chloride and potassium), the electrical potential acoss the cell membrane must exactly balance the concentration gradient for both ions
- Because the membrane potential can only have one value , his equilibrium condition will be satisfied only when equilibrium potentials for both ions are equal
- Concentration of chloride ions will be 125
- Then multiply that by 5 to et 625 , and take the square roote to get 25 which means total ion concentration is 50
- protein concentration is 200
- Donnan equilbirum specifies conditions that must be met if two ions that can cross a cell membrane are simultaneously to be at equilibrium across that membrane
Action potential Charecteristics
- Ot os all or none:it will either occur or not occur
- It is self-propagating:each region of depolarization serves to generate action potentials on either side
- It is non-decremental: it doesn ot decrease in strength
- Once you start,won’t stop till you reach the end ( end of the axon)
- Non-decremental:does not decrease in strength
Ion channels related to Action potential propagation
- Ion channels are channesl that allow the passage of ions from one side of the membrane to the other. They are typically very selective ,allowing only one kind of ions to pass through
- They may always be open(such as slow-leak channels)
- They may be gated, only opening when certain conditions are met
- Ligand gated:involves the attachment of a chemical messenger such as a neurotransmitter or hormone to a receptor
- Voltage gated:involves a change in the membrane potential
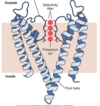
Molecular strucutre of voltage gated sodium channels
- Channel consists of four domains
- Four domains are thoguht to be arranged in a cylindrical configuration
- Each domain has six hydrophobic transmembrane segments(s1-S6)
- The S4 segment within each domain has a high positive charge
- Inactivation gate is associated with an intracellular hydrophilic linkage between domains three and four
Voltage gated sodium channels
- Have two gates
- Activation gate
- inactiviation gate
- Activation gate is closed and the inactivation gate is opened at -90mV(with the inside of the axon membrane negative relative to the outside)
- Both gates are opened between -90mV to +35mV
- Activation gate opens as voltage reaches -70mV to -50mV
- Activation gate is opened and the inactivation gate is closed at +35mV to -90mV
Voltage Gated Potassium Channels
- Potassium Channels have channel diamters that are too small for hydrated potassium and hydrated sodium ions
- Near the entrance to the channel there are loop from the pore helix that are bound to carbonyl oxygens
- This forms a selectivity filter
- Smaller hydrated ions such as sdoium are not affected by the selectivity filter but are too large to pass through the potassium channel
- Larger hydrated ions such as potassium will be dehyrated by the selectivity filter, allowing the smaller”naked” potassium ion to pass through the channel
- Single gate closed at a resting potential of -90mV
- slow activation opens the gate from +35mV to-90mV
- No ions are passing through gated channls during resting potential
Steps in an action potential on a neuron axon membrane
- Resting Stage
- -90mV
- Depolarization stage
- Membrane suddenly becomes permeable to sodium ions
- Membrane potential may overshoot for large axons
- Repolrazation stage
- Sodium channels close within a few 10,000ths of a second
- Potassium channels open more than normal
- Sodium and potassium conductance
- inside is going to become more positive and need potential to repolarize

Action potentials
- Current flowign down the insdie of an axon at a particular point can continue down the interior of the fiber or cross the membrane at that point. Usinv either of these two alternatives,the speed at which hte action potential travels(is propagated) can be icnreased
- Increasing the diamter of the axon: utilized by invertebrates(especially cephalopods such as squids) but also plays a factor in vertebrate axons of various diamters
- Large diamter axons offer larger cross-sectional area to internal flow of current
- Current has many alternative paths to follow and resistance is lowered and the action potential speed is increased
- Membrane permeability and the cross sectional diamter ways to increase speed and true of any conductive cell
- not trying to force current through narrow gauge wire