Membrane Proteins and the Secretory Pathway Flashcards
(46 cards)
four types of membrane proteins
transmembrane, monolayer-associated, lipid-linked, protein-attached
Type I topology
single pass protein anchroed to the lipid membrane with a stop transfer anchro sequence, have their N-terminus targeted to the ER lumen
Type II topology
single pass protein anchored with a single-anchor sequence and hte carboxyl terminus in the ER lumen
Type III topology
single pass protein with a signal-anchor sequence and the amino terminus in the ER lumen
Type IV topology
multi-pass protein, amino terminus on either side
glycosylphosphatidylinositol (GPI) anchor
modified lipid in cell membrane that cells adhere to
some type I proteins exchange their carboxyl terminal transmembrane segment for a GPI anchor
Describe the directions of vesicular traffic in cells.

protein transport into ER
signal recognition particle (SRP) recognizes signal sequence and brings it to receptor in the ER membrane
protein goes through channel, and signal is eventually cleaved by a signal peptidase
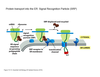
What are some different ways to orient proteins based on signal sequence?

N-linked glycosylation
the process of adding sugar molecules onto all proteins in the ER membrane, same sugars
important for protein stability

BiP
one of many ATP-dependent chaperones that assist folding as the protien emerges from the translocon
Calnexin
calcium binding protein that monitors that folding of glycoproteins
binds to oligosaccharides and “senses” when the protein is correctly folded
mannosidases
trim mannose residues from the oligosaccharide, function similarly to calnexin to ensure proper folding
protein disulfide isomerases (PDIs)
catalyze formation of disulfide bonds
ERP57
chaperone protein that works with calnexin to ensure proper folding of proteins using the trimming of the oligosaccharides as a guide
SEL1
protein in the ER that pushes improperly folded proteins out as a single chain, which is then polyubiquitinylated and degraded
PERK
protein kinase that phosphorylates eIF2alpha, which blocks translation
ATF4 can escape the transtion block so it goes to the nucleus and activates a stress response
ATF6
released from the ER membrane by high levels of BiP, and goes through the golgi to get cleaved and become a transcription factor to activate stress genes
IRE1
BiP interaction activates a unique splicing mechanism, causes intron mechanism for XBP1 to be inactivated, creating a transcription factor to activate a stress response
also responsible for triggering autophagy or apoptosis through activation of Jun Kinase
unfolded protein response
a collection of several mechanisms the cell can undergo to deal with unfolded proteins
uses BiP as a sensor

ER-associated degradation (ERAD)
a process of degradation that proteins undergo if proteins cannot fold properly or take too long to fold in the ER, uses SEL1 to unfold proteins and mark for degradation
functions of the cis golgi network
sorting, phosphorylation of oligosaccharides on lysosomal proteins
functions of the cis cisterna of golgi
removal of mannose
function of mdial cisterna of golgi
removal of mannose and addition of N-acetyl-glucose (GlcNac)








