Unit 6 - Apoptosis, advanced therapies and concepts of immuno-oncology Flashcards
(107 cards)
4 reasons why cells die
- when they get old e.g. lifespan of RBC = 120 days
- irreversible damage - exposed to extensive damage e.g. ischaemia, stress e.g. pathophysiological conditions, fever
- when they become superfluous - tissue/organ development e.g. loss of interdigital web cells, loss of tadpole tail; elimination of superfluous immune cells after recovery from infectious disease
- when they become dangerous to the body/organism - virus infected cells, malignant/cancer cells
how do cells die
apoptosis - highly regulated, organised form of cell death
necrosis - dysregulated form of cell death
characteristics of apoptotic cells
programmed form of cell death
cell separates from neighbouring cells
cell shrinkage
membrane blebbing
chromatin condenses and the nucleus fragments
cell breaks up into apoptotic bodies which are phagocytosed, such that cellular components or waste products do not produce an inflammatory response
predictable, reproducible sequence of events

key differences between apoptosis and necrosis
APOPTOSIS
- single cells die
- cell shrinks
- remnants are phagocytosed
- neat, controlled
- no trace left
- no inflammation follows
NECROSIS
- chunks of tissue die
- cells swell and burst
- remnants are not cleared
- cell content released into EC space
- inflammation follows

too little apoptosis
malignant and pre-malignant conditions
lymphoproliferative disorders
leukemias
lymphomas
solid tumours
too much apoptosis
alzheimer’s
parkinson’s
stroke
atherosclerosis (CV)
ischaemia/reperfusion injury (CV)
dysentery (intestinal)
diarrhea (intestinal)
phases of apoptosis
INITIATION
the cell makes the decision to kill itself
EXECUTION
cell commits itself to die and activates the machinery for cellular disassembly
CLEARANCE
the apoptotic cell/bodies are removed from the system
what initiates apoptosis
appearance of death signals
withdrawal of survival factors
categories of death signals that initiate apoptosis
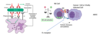
EXTRINSIC
death signal can derive from environment of cell
hormones and cytokines e.g. death ligands secreted by immune cells can kill infected/cancerous cells
INTRINSIC
can derive from inside of cell
overwhelming stress/irreparable damage (DNA damage, hyperthermia, exposure to toxic compounds)
withdrawal of survival factors - initiate apoptosis
most cells in the body depend on the presence of growth factors
in their absence, apoptosis is initiated and cells die
e.g. immune cells depend on presence of IL e.g. T cells on IL-2 and IL-15
what are caspases
effectors of apoptosis
cysteine dependent aspartic acid specific proteases
co-ordinate destruction of cellular structures
hallmark of apoptosis - required
proteases
enzymes that catalyse the breakdown of proteins into smaller polypeptides or AAs
cysteine dependent
active site of the caspase contains cysteine residue that is required for its catalytic activity
aspartic acid specific
cleave substrate proteins at aspartic acid residues
how are caspases synthesised
as inactive precursors (pro-caspases)
2 groups of caspases
initiator - 8, 9, 10
effector - 3, 6, 7
initiator activate effector, which then mediate apoptosis through the proteolytic cleavage of 1000s of proteins
initiator caspases
8
9
10
effector caspases
3
6
7
extrinsic activation pathway is also known as
death receptor mediated pathway
intrinsic activation pathway is also known as
mitochondrial mediated pathway
death receptors involved in extrinsic pathway
when are they expressed
family involved
subset of TNFR superfamily - TNFR1, Fas, TRAIL-R1, TRAIL-R2
some receptors are constantly present on cell surface, while other are expressed only upon damage
external cysteine rich domain - involved in ligand binding
transmembrane domain
internal death domain (DD) - needed for binding of adapter proteins like FADD
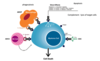
MOA of extrinsic pathway
death receptors e.g. TNFR, FasR are transmembrane receptors present on cell surface
binding by death ligands e.g. Fas causes death receptor to oligomerise
death receptors change shape and recruit adaptor molecules e.g. FADD or TRADD
several pro-caspase-8 molecules recruited and transactivate each other
active caspase-8 (initiator caspase) cleaves other caspases promoting irreversible cascade and cell death
DISC - Death Inducing Silencing Complex

intrinsic cell death pathway
when is it activated
key event
family involved
activated in response to a variety of internal stresses including DNA damage, ER stress, growth factor deprivation
release of mitochondrial intermembrane space proteins is the key event in intrinsic cell death
mitochondrial mediated release of intermembrane space proteins is controlled by the BCL-2 family