Exam 2 Flashcards
What kind of cell is this?

Gram positive
What kind of cell is this?

Gram negative
What color are gram positive bacteria?
Purple
What color are gram negative bacteria?
pink
Which bacteria has a thicker cell?
gram negative
Which bacteria has porins?
gram negative
Where are beta lactamases located in gram positive bacteria?
External space, thus you need to create larger quantities
where are beta lactamases located in gram negative bacteria?
Within in the cell in periplasmic space since it can go through porins
What is the main barrier keeping drugs out of the cell in gram positive bacteria
Bacterial membrane
How many membranes does gram positive bacteria have
1
How many membranes do gram negative bacteria have
two –> inner and outer membranes
What is in gram negative bacteria’s peptidoglycan and how is it cross-linked?
- meso-diaminopimelic acid residue (DAP)
- peptidoglycan is cross-linked by a bridge between the DAP residue of one strand and the terminal D-Ala of another
What is in gram positive bacteria’s peptidoglycan and how is it cross-linked?
- L-lysine residue
- Bridge exists between the L-Lys strand and the terminal D-Ala of the second molecule
What is the enzyme that cross-links the peptidoglycan strands?
transpeptidases
beta lactam antibiotic mechanism of action
- inhibition of transpeptidases that glue the peptidoglycan strands together by cross-linking
- beta lactam antibiotics acylate the transpeptidase Ser residue in the enzyme active site to form stable product, which inactivates the enzyme, inhibiting peptiodglycan cross-linking, which results in a defective bacterial cell wall
What is the reactivity of the beta lactam system due to
- highly strained four-membered ring
Bacterial transpeptidases and catalyzation reactions of host cells
Bacterial transpeptidases do not catalyze reactions with host cell proteins because the bacterial substrate contains unnatural D-Ala amino acid residues that are not found in the host cell proteins
How can resistance to beta lactam antibiotics occur (4)
- decreased cellular uptake of the drug
- mutation of the penicillin binding proteins to decrease their affinity for penicillins
- presence of an efflux pump that pumps the antibiotic out of the cell
- induction or elaboration of bacterial beta lactamases
Rate of hydrolysis of the actylated beta lactamase
Fast, so the enzyme can hydrolyze many drug molecules rapidlyHyd
How much of the US population is allergic to beta lactam antibiotics?
6-8%
How does allergenicity of beta lactam antibiotics occur
Drugs acts like a hapten and acylates host cell proteins, which then raise antibodies that result in an allergic reaction
Cross reactivity in mild reaction of beta lactams
Cephalosporin or carbapenen can be tried since cross-reactivity is 5-15%
Cross reactivity in severe reaction of beta lactams
Cephalosporins and carbapenems are avoided, but aztreonam can be used
*Under the acidic conditions, the main degradation of Pen G are:
- Benzylpenicillenic acid
- Benzylpenillic acid
- Benzylpenicilloic acid
uses achimeric assistance
*Product of penicillin degradation under basic conditions
penicilloic acid
Antibiotic activity of penicillin hydrolysis products
no antibiotic activity
Is the hydrolysis of the beta lactam reversible or irreversible
Irreversible
Electronegative substituents and nucelophilicity of beta lactams
- Electronegative substituets on the side chain carbonyl reduce the nucleophilicity of the chain amide carbonyl oxygen atom
- This stabilizes the penicillin against hydrolysis under acidic conditions, since tgeh first step in the hydrolysis reaction is decelerated
Which medication is more stable to hydrolysis in the stomach and why? Penicillin V vs Penicillin G

Penicillin V is more stable to hydrolysis in the stomach than Penicillin G because the electronegativity of the ether oxygen dereases the nucleophilicity of the amide carbonyl
What catalyzes penicillin degradation reactions
Heavy metal ions- therefore keep away from penicillin solutions
Lipophilic side chains and protein bound relationship
penicillins with more lipophilic side chains are more highly protein bound
protein binding and bioavailability relationship
protein binding reduces bioavailability by reducing the effective concentration of the free drug
protein binding and degradation relationship
protein binding in general protects drug from degradation since they dont react with hydrophilic enzymes
what is rate-limit half life of penicillin
- Renal excretion rate
- half-lives of penicillins are generally not affected by protein binding, since their dissociation rates from the protein are fast
penicillin excretion routes
- rapidly excreted by the renal or biliaqry routes
- 10% of renal excretion is by glomerular filtration
- 90% is by tubular secretion
How does kidney disease or failure effect half-lives of penicillins
Half-lives are prolonged
Tubular secretion MOA of pencillins
The penicillins are anionic and competition with the anion probenecid for the secretion mechanism causes an increase in half life when probenecid is administered along with the penicillin
Dose range of penicillin
3-12 g per day for an average adult
Serum half-lives of penicillin
Serum half-lives are generally 0.5 to 2 hours
Penicillin G antimicrobial spectrum
Gram + cocci
N. gonorrhoeae and H. influenza
Pencillin beta lactamase sensitivity
Yes
Penicillin G administration
Orally in large doses, although the most effective route is parenteral
Penicillin G toxicity
Acute allergic reactions
Penicillin G precautions
Pen G should be used with caution in individuals with histories of signficant allergies and/or asthma
Penicillin Notes
Pen G is the drug of choice for treatment of more infections than any other antibiotic
How are synthetic penicillins made by
acylation of 6-APA
What structure is this?

penam
what structure is this?

penem
what structure is this?

carbapenem
what structure is this?

cephem
what structure is this?

monobactam
benylpenicillin benzathine and benzylpenicillin procaine PK
- because of low solubility, the drug is released slowly from the intramuscular injection site
- duration of action is longer and the blood levels are than with other parenteral penicillins
benylpenicillin benzathine and benzylpenicillin procaine administration
- Should only be administered by deep IM injection
- Inadvertent IV administration can result in cardiac arrest and death
- Injection near a nerve can result in permanent neurological damge
benylpenicillin benzathine and benzylpenicillin procaine therapuetic use
- moderately severe to severe infections of the upper-respiratory tract, scarlet fever, and skin and soft tissue infections due to susceptible streptococci
- Moderately severe pneumonia and otitis media due to susceptible pneumococci
Difference between penicillin V and penicillin G
- Pencillin V is more stable in acid
- the increase in stability in acid attributed to the electronegative ether oxygen which decreases the nucleophilicity of the side chain amide carbonyl and therefore decreases its participation in the beta lactam hydrolysis reaction
Does methicillin have beta lactamase sensitivity and why
No because of steric hindrance of nucleophilic attack by the enzyme on the beta lactan carbonyl
Is methicillin stable to acid
No because of electron donation toward the amide carbonyl oxygen by the o-methyoxygroups, making the amide carbonyl oxygen more nucleophilic
How is MRSA resistant to methacillin
- Mutation in a pencilin binding protein (transpeptidase)
- The gene coding for this protein is called methicillin resistance gene (mecA), and the penicillin binding protein that it codes for is PBP2A
Does nafcilin have beta lactamase sensitivity
No
Nafcillin stability in acid
Slightly more stable than methicillin in acid, but is clinically identical to methicillin
What drug is this?

oxacillin
what drug is this?

cloxacillin
what drug is this?

dicloxacillin
Does oxacillin, cloxacillin, and dicloxacillin have beta lactamase sensitivity?
No
Is oxacillin, cloxacillin, and dicloxacillin preferred treatment for septicemia and why?
No because they are highly protein bound
xacillin, cloxacillin, and dicloxacillin cross resistance
cross-resistant with methicillin
Ampicillin antimicrobial spectrum and how
- Many gram (-) microorganisms are sensitive, including Salmonella, shegella, proteus mirabilis, e. coli, h. influenza, and n. gonorrhoeae
- the inner surface of porirns in hydrophilic and porins all transport ionic compounds
- the charged amino group of ampicillin at physiological pH allows ampicillin to be transported into gram (-) bacteria through the porins
Is ampicillin stable in acid and why
- Yes because the amino group is protonated in the stomach, so the positively charged nitrogen is more electron-attracting
- This decreases the nucleophilicity of the amide carbonyl oxygen so that it does not participate in ring-opening of the lactam
Does amoxicillin or ampicillin have better absorption and why
Amoxicillin because it is an analog of ampicillin in which a phenolic hydroxyl group has been introduced into the aromatic ring
MOA of clavulanic acid and sulbactam
acylate the serine hydroxyl group in the active site of the beta lactamase to form a reactive intermediate that then inactivates the ezyme irreversibly
Does azlocillin, mezlocillin, and piperacillin have stronger or weaker potencies and why
Enhanced potencies because the added side chain fragments resemble a longer section of the peptidoglycan chain than ampicillin
How to treat piperacillin, resistant bacteria
Tazobactam
What is the main starting material for cephalasporins
cephalasporin c
what can cephalapsorin C be converted to to make a chemically useful cephalasporn
7-aminocephalosporic acid
Cephalosporin MOA
- Reaction with transpeptidases (penicillin-binding proteins) results in inhibition of peptidoglycan cross linking
- Many cephalosporins contain leaving groups that faciitate beta lactam ring opening
Hydrolysis of cephalosporins
Cephalosporins are hydrolyzed by beta lactamases
How does cephalosporin resistance occur
Cephalosporins are hydrolyzed by beta lactamases, rendering them inactive
beta-lactamase specificity
- There are over 340 different beta lactamases known
- They can be specific for certain antibiotics and classes of antibiotics
Allergenicity of cephalosporins
- allergic reactions are less common and less severe than with penicillin
cross allergenicity of cephalosporins
since allergenicity is common, cephalosporins should be used with caution, if at all, in patients who are allergc to penicillins
What medications are first generation cephalosporins
- Cefazolin
- cephanlexin
what are first generation cephalosporins primarily active against
- gram + cocci: staph aeureus and staph pyogenes
- group b streptococci: s agalactiae and s. pneumoniae
If the cephalosporin substituents at C-3 are chemiccaly reactive, how are they administered?
Parenterally
If the cephalosporin substituents at C-3 are not chemiccaly reactive, how are they administered?
orally
what medication is this and How is this medication administered?

cefazolin; parenteral
what generation is cefazolin
first generation
What is this medication how is this medication administered?
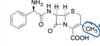
cephalexin; orally
What generation is cephalexin
first generatio
how is cephalexin able to confer oral activity
- Substituent at C-3 is not chemically reactive
- Contains an ampicillin-type side chain at C-7 that makes it more stable and helps to confer oral activity
what do second generation cephalosporins target
- Retain the anti gram (+) activity of the first generation and h. influenzae as well
- Some gram (-) activity including some strains of acinetobacter, citrobacter, enterobacter, e. coli, klebsiella, neisseria, proteus, providencia, and serratia
what generation if cefuroxime
second
What is special about cefuroxime
can be given parenterally and orally
Difference between syn and anti methoximino groups
- *The syn methoximino group is more resistant than the anti isomer
- the syn isomer can be photochemically isomerized to the anti isomer in solution to for a 1:1 mixture of syn and anti isomers
Third generation cephalosporin activity
- less active against staphlocci than the first generation agents
- much more active vs gram (-) bacteria than either the first or second agents
what organisms are sensitive against third generation cephalosporins
- morganella
- bacteroides fragilis
- pneudomonas aeruginosa
- some enterobacteria
What is on almost all of the third generation cephalosporin structures
an aminothiazole substituent and contain an oxime ether at the 7-position
what drug class does ceftazidine belong to?
third generation cephalosporin
What moiety at C-7 conveys enhanced stability vs beta lacatamases
large oxime ether moiety
what drug class is cefixime
third generation cephalosporin
fourth generation cephalosporin antibacterial spectrum
retain the antibacterial spectrum of the third generation cephalosporins and also add pseudomonas aeruginosa and some enterobacteria that are resistant to third generation cephalosporins
more active against gram positive organisms
cefepine drug class
fourth gen cephalosporin
route of administration of cefepime
parenteral
what drug class is ceftolozane
fourth gen cephalosporin
what drug is ceftolozane combined with and what does it treat
- Tazobactam
- effective against many bacteria that are resistant to other antibiotcs
- treats both gram positive and gram negative bacteria
what drug class is ceftraoline
fifth generation cephalosporin
MOA of ceftraline fosamil
ceftraline fosamil is a prodrug that is hydrolyzed metabolically via phosphatase after IV infusion to ceftraroline
Ceftaroline spectrum of activity
broad-spectrum, fifth-generation cephalosporin antibiotic that is active vs MRSA and is used vs MRSA and community acquired bacterial pneumonia since it can inhibit the MRSA PBP2a
what drug class is cefiderocol
fifth gen cephalosporin
cefiderocol indication
complicated UTI
cefideroocol MOA
transpeptidase inhibitor
what structual unit does cephamycins have that make them special
7-alpha methoxyl group
cefoxitin spectrum activity
broad spectrum of gram positive and gram negative bacteria
MOA of cefoxitin
beta lacatamase inducer
cefoxitin drug class
cephalosporin
cefotetan drug class
cephalosporin
Caution point of cefotetan
releases N-methylthiotetrazole, which can cause hypothrombinemia, and can also cause a reaction to ethanol that is similar to disulfuram
why can’t thienamycin be used as a drug and how has this been overcame
- thienamycin is too reactive to be used as a drug, since the primary amino group attacks the beta lactam intermolecularly
- Been overcame by removing n-formiminoyl group to create imipenem
Why are carbapenems more reactive than penicillins
- The sulfur that is present in the thiazolidine ring of the penicillins is replaced by a methylene
- This increases reactivity because a methylene is smaller than a sulfure, so the ring strain is greater in the carbapenems
MOA of imipenem
- reacts with penicillin binding proteins
- reacts with and inhibits beta lactamases
what is imipenem hydrolyzed by
renal dehydropeptidase-1, but this can be overcome by co-administration of the dehydropeptidase-1 inhibitor cilastatin
what is cilastatin and imipenem spectrum of activity
active against both gram + and gram - bacteria: used to treat of the gut, GI tract, bone, skin, and endocardium
meropenem drug class
carbapenem
does meropenem have to be administered with cilastatin
no because the 1-beta-methyl group confers stability to dehydropeptidase-1
why does ertapenem have an extended half life
highly protein bound so that it allows it to be administered iv once every 24 hours
aztreonam drug class
monobactams
origin of aztreonam
- aztreonam disodium is totally synthetic but the design was inspired by monocyclic beta lactam natural products called monobactams
- sulfamic acid group takes place of the c-2 carboxyl group in the penicillins and cephalosporins
- electronegativity of the sulfamic acid activates the beta lactam ring toward hydrolysis and to reaction with penicillin-binding proteins
- sulfamic acid group takes place of the c-2 carboxyl group in the penicillins and cephalosporins
aztreonam cross allergenicity
cross allergenicity with penicillins and cephalosporins has not been reported except for ceftazidine, which has an indentical oxime ether sidechain
what drug is this?

vancomycin
what drug is this?

teicoplanin
vancomycin mode of action and what makes it different from penicillins
- vancomycin involves binding to the peptidly side chain d-alanyl-d-alanyl terminus in the peptidoclycan precursor (before cross-linking)
- transpeptidase reaction that is required for cross-linking is inhibited by the high affinity binding of vancomycin to the the substrates
- vancomycin also inhibits the transglycosylation step in peptidoglycan synthesis, which penicillins do not do
vancomycin spectrum of activity
primarily bactericidal and is active against gram (+) bacteria
why is vancomycin not effective against gram negative bacteria
vancomycin is too big to get through the porins
how has vancomycin resistance occurred
- Mechanism of resistance appears to be mutation of the peptidoglycan cell wall precursor from D-Ala-D-Ala to D-Ala-D-lactate
- vancomycin does not inhibit the transpeptidase when the substrate is d-ala-d-lactate because vanomycin has 1000 times less affinity for the d-ala-d-lactate precursor
vancomycin PK and distribution
- vancomycin does afford appreciable blood levels after oral administration and is usually administered IV
- vancomycin is highly distributed and 90% eliminated by glomerular filtration with a half life of 4-11 hour
vancomycin therapeutic use
- given orally to treat c. diff
- strep-induced endocarditis
- MRSA
- MRSE
vancomycin toxicity and effects
- hypersensitivity
- nephrotoxicity
- ototoxicity
what drug is this?

oritavancin
oritavancin drug class
semisynthetic lipoglycopeptide antibiotic
oritavancin moa
inhibits transpeptidation and transglycosylation, which disrupts the membrane of gram-positive bacteria
oritavancin spectrum of activity
gram positive bacteria-MRSA skin infections
telavancin drug class
lipoglycopeptide antibiotic
what drug is this

telavancin
telavancin MOA
like vancomycin, telavancin binds to the D-Ala-D-Ala terminus of the peptidoglycan in the growing cell wall by inhibiting transpeptidation and transglycosylation
telavanvin therapeutic use
MRSA and other gram positive infections
what drug is this

dalbavancin
dalbavancin drug class
second generation lipoglycopeptide antibiotic
dalbavancin mechanisms
Identical to vancomycin: binds to the D-Ala-D-Ala residue on growing peptidoglycan chains and prevents transpeptidation and transglycosylation from occurring, thus preventing peptidoglycan elongation and cell membrane formation
what drug is this

daptomycin
what drug class is daptomycin
lipopeptide antibiotic
daptomycin moa
aggregation of daptomycin in the bacterial membrane creates holes that leak ions
daptomycin therpeutic use
used parenterally to treat systemic infections caused by gram-positive bacteria, including MRSA
What structure is this

penicillin
what structure is this

cephalosporin
what structure is this

monobactam
what structure is this

carbapenem
what are the four classes of beta lactams
- penicillins
- cephalosporins
- monobactams
- carbapenems
beta lactam MOA
inhibit cell wall synthesis
beta lactam mechanisms of resistance
- beta lactamase degradation
- PBP alteration
- Decreased penetration
Are beta lactams bacterialstatic or bacterialcidal and in what kind of manner
bactericidal in a time dependent manner
average half-life of beta lactams
less than 2 hours
how are beta lactams eliminated
primarily eliminated unchanged by the kidneys
is cross allergenicity possible in beta lactams
yes, except for aztreonam
what do all penicillins share
a beta lactam ring attached to a 5-membered thiazolidine ring
penicillin mechanism of action
interfere with cell wall synthesis by binding to and inhibiting penicillin-binding proteins PBPs located in bacterial cell walls
what does inhibition of PBPs lead to
inhibition of final transpeptidation step of peptidoglycan synthesis
Why are beta lactamases problematic for penicillins
The enzyme hydrolyzes the beta lactam ring, which inactivates the antibiotic
what gram positive bacteria produces beta lactamases
penicillin resistance staph. aureus
what gram negative bacteia produce beta lactamase enzymes
- h. influenzae
- moraxella catarhalis
- n. gonorrhoeae
- e. coli
- klebsiella pneumoniae
- enterobacter spp
what gram negative anaerobes produce beta lactamase
bacteroides fragilis
how are PBPs lead to penicillin resistance
- Alteration in structure of PBPs leading to decreased binding affinity
PBP Resistance Examples
MRSA and PRSP via that mECA gene
How do porins cause penicillin resistance
alteration of outer membrane porin proteins leading to decreased penetration
why were semi-synthetic penicillins developed
to provide enhanced antibacterial activity
what are the natural penicillins
- aqueous penicillin G
- benzathine penicillin G
- procaine penicllin G
- phenoxymethyl penicllin (penicillin VK)
natural penicillins are the drug of choice for what bacteria
treponema pallidum
what are penicillinase-resistant penicillins also known as
antistaphylococcal penicillins
what are the parenteral penicillinase-resistant penicillins
naficillin and methicillin (no longer used)
what is the oral penicillinase-resistant penicillins
dicloxacillin
what are penicillinase-resistant penicillins sensitive toward
methicillin-susceptible s. aureus (MSSA)
why were aminopenicillins developed
in response to the need for agents with some gram-negative activity
parenteral aminopenicllin
ampicillin
oral aminopenicillins
ampicillin and amoxicillin
what are aminopenicillins
semi-synthetic derivative of natural penicillin with the addition of an amino group
what is ampicillin the drug of choice for
enterococcus spp
what gram positive bacteria does aminopenicillin enhance activity against
listeria monocytogenes
what gram negative bacterias does aminopenicillin enhance activity against
- SHEP
- salmonella/shigella
- h. influenzae BL
- e. coli (some)
- proteus mirabilis
why were carboxypenicllins developed
in response to the need for agents with enhanced activity against gram negative bacteria
carboxypenicllin structure
semi-synthetic derivatives of natural penicillin with the addition of a carboxyl group
parental carboxypenicllin
ticarcillin
do carboxypenicllins have increased activity against gram positive aerobes
marginal
what gram negative aerobes do carboxypenicllins cover
- SHEPMEPP
- salmonella/shigella
- h. influenza BL+
- e. coli (some)
- proteus mirabilis
- morganella
- enterobacter
- pseudomonas aeruginosa
- proteus mirabilis
what penicllin do you use to treat MSSA
naficillin
what medication do we use to treat syphillis
IM benzathin penicllin
why were ureidopenicillins developed
in response to the need for agents with even more enhanced activity against gram-negative bacteria
ureidopenicillin structure
semi-synthetic derivatives of the amino-penicllins with acyl side chain adaptations
ureidopenicllin parenteral agent
piperacillin
what additionnal gram negative aerobes do ureidopenicillins cover
- serratia marcescens
- some klebsiella spp
MOA of beta lactamase inhibitors
irreversibly bind to catalytic site of beta lactamase enzyme
Parental beta lactamase inhibitor combinations
Unasyn and Zosyn
Unasyn component
ampicillin-sulbactam
Zosyn
Piperacillin-tazobactam
oral beta lactamase inhibitor
augmentin
augmentin components
amoxicillin-clavulanate
target organism of beta lactamase inhibitor combos
bacteroides spp
What bacterial killing depenedent on in penicllins
time
what correlated with efficacy of penicllins
time above MIC
Goal of penicllin dosing
Administer agents to maintain serum concentrations > MIC of infection bacteria for 50% of dosing interval
Many penicllins are degraded by what
gastric acid
Lower concentrations achieve with PO versus IV so that oral penicllins should only be used for mild to moderate infections
Two best orally available penicillins
PEN VK and Amoxicillin
Do penicillins have csf permeabilit
Adequate CSF concetrations of penicillins (but NOT beta lactamase inhibitors) are achieved ONLY in the presence of inflamed meninges with high-dose parenteral administration
Penicllin primary elimination
most are eliminated unchanged by the kidney so that dosage adjustment is required in the presence of renal insufficiency; probenacid blocks tubular secretion
penicllin elimination exceptions
nafcillin and oxacillin are eliminated primarily by the liver- do not require adjustment in renal insufficiency
Why is sodium load in penicllin cause issues?
High contents of sodium must be used in caution in patients with CHF or renal insufficiency because of electrolyte abnormalities and fluid retention
Penicllins with sodium content (5)
- Sodium Penicllin G
- Nafcillin
- Carbenicillin
- Ticarcillin
- Piperacillin
Sodium Penicllin G sodium content
2.0 mEq per 1 million units
Nafcillin sodium content
2.9 mEq/gram
Carbenicllin sodium content
4.7 mEq/g
ticarcillin sodium content
5.2, which is the highest
piperacillin sodium load
1.85 mEq/gram
Which penicllin has the highest sodium load
ticarcillin
Natural penicillin clinical use
potential drug of drug for syphillis
penicillinase-resistant penicillins clinical uses
MSSA
augmentin clinical uses
sinusitis and otitis media, bite wounds
Unasyn, Zosyn, Timentin clinical uses
polymicrobial infections
Zosyn clinical use
empiric therapy for febrile neutropenia or hospital acquired infections
cross reactivity of penicillins
cross reactivity exists among all penicllins and even some other beta lactams
two main adverse effects of penicllins
neurologic and hematologic
how does penicllins cause neurologic adverse effects
direct toxic effect, especially in patients receiving high IV doses in the presence of renal insufficiency
seizures are common
hematolic penicllin adverse effects
netropenia and thrombocytopeni occur usually during prolonged therapy greater than 2 weeks but reversible upon discontinuation
GI adverse effects of penicllins
increased lfts, nausea, vomiting, diarrhea, C. diff
what is interstitial nephritis
immune mediated damage to renal tubules characterized by an abrupt increase in serum creatinine, eosinophilia, and eosinophiluria
what agents cause interstitial nephritis
methicillin or nafcillin
what classes fall under beta lactams
- penicillins
- cephalosporins
- monobactams
- carbapenems
what do cephalosporins contain
- contain a beta lactam ring attached to a 6-membered dihydrothiazine ring, which confers greater stability against some beta lactamase enzymes
how are cephalosporins active against anaerobes
cephamycins have methoxy group at C-7 and are active against anaerobes
cephalosporin mechanism of action
interfere with cell wall synthesis by binding to penicillin-binding proteins located in bacterial cell walls
how does cefiderocol enter the bacterial membrane
Acts as a siderophere by binding to free ferin that enters the bacterial cell well
What does inhibition of PBPs lead to
inhibition of the final transpeptidation step of peptidoglycan synthesis, which exposes a less osmotically stable cell wall that leads to decreased bacterial growth, lysis, and death
T/F: cephalosporins are bactericidal
true
cephalosporin mechanism of resistance
production of beta lactamase enzymes: most important and most common where enzyme hydrolyzes beta lactam ring causing inactivation
what is the one cephalosporin that is active against MRSA
cefteroline
which cephalosporin generation are the most active verses gram positive aereobes
first generation
how do you lose gram positive activity and increase in gram negativity within cephalosporin generations
Lose gram-positive activity with an increase in gram negative activity as you go from 1st -> 2nd -> 3rd to 4th
how does beta lactamase activity change with cephalosporin generations
greater beta lactamase stability as you go from 1st -> 2nd -> 3rd -> 4th
what are the two main first generation cephalosporins
cefazolin and cephalexin
what gram positives are first gen cephalosporins active against
- pen-susc S. pneumoniae
- meth-susc S. aureus
what gram negatives are first generation cephalosporins are active against
p. mirabilis
e. coli
k. pneumoniae
what additional activity do several second generation cephalosporins have
several second generation agents have activity against anaerobes (the cephamycins)
are first or second generation cephalosporins better for treating meth-susc S. aueres
first generations
what gram negatives do second generation cephalosporins cover
- p. mirabilis
- e. coli
- k. pneumoniae
- h. influenzae
- enterbacter spp
- neisseria spp
- m. catarrhalis
what anaerobes are second geneneration cephalosporins active against
bacteroides fragilis
what are the three common second generation cephalosporins
- cefuroxime
- cefprozil
- cefoxitin
what are the three most common third generation cephalosporins
- ceftriaxone
- ceftazidime
- cefpodoxime
third generation cephalosporins are active against what gram negative aerobes
- p. mirabilis
- e. coli
- k. pneumoniae
- h. influenzae
- m. catarrhalis
- n. gonorrhoeae
- n. meningitidis
- citrobacter
- enterbacter
- acinetobacter
- morganella
- serratia
- providencia
- salmonella
- shigella
what third generation cephalosporins are active against pseudomonas aeruginosa
ceftazidime and cefoperazone
does ceftriaxone have activity against pseudomonas aeruginosa
NO
What are the three third generation cephalosporins
- ceftriaxone
- ceftazidime
- cefpodoxime
what is the only fourth generation cephalosporin available
cefpime
what are fouth generation cephalosporins poor inducers of
poor inducer of inducible AmpC beta lactamse enzymes
what is the anti-MRSA cephalosporin
ceftaroline
ceftaroline does or does not cover pseudomonas aeruginosa
does not
cefiderocol spectrum of activity
many ESBLs, AmpCs, CREs
what is ceftolozane and tazobactam sensitive to
pseudomonas aeruginosa
Overall, cephalosporins are NOT active against (6)
- MRSA (except ceftaroline)
- Enterococcus spp
- listeria monocytogenes
- stenotrophomonas maltophilia
- clostridium difficile
- atypical bacteria, including legionella
which cephalosporin is considered a potential drug of choice for infections due to MSSA
cefazolin
which cephalosporin does NOT have activity against pseudomonas aeruginosa
ceftriaxone
what is cephalosporins bactericidal activity dependent on
Time –> Time > MIC is PD parameter that correlates with efficacy
cephalosporins absorption
oral cephalosporins are well absorbed, but achieve lower serum concentrations than parenteral products; food decreases the absorption of cefaclor and loracarbef
cephalosporins distribution
- Widely distributed into tissues and fluids
cephalosporins CSF distribution
CSF concentrations achieved ONLY with PARENTERAL cefuroxime, 3rd and 4th generation agents
cephalosporin primary elimination
most are primarily eliminated unchanged by the kidney via glomerular filtration and tubular secretion; dosage adjustment of these agents is required in the presence of renal insuffiency
what cephalosporins are not eliminated by the kidney
- ceftriaxone (biliary)
- cefoperazone (liver)
what is the half life of most cephalosporins
< 2 hours
what is the notable half life of cephalosporin
most cephalosporins have short elimination half-lives (<2 hours)- on noteable exception is ceftriaxone whose half-life is 8 hours
ceftaroline clinical use
skin and soft tissue infections including those caused by MRSA
can you use caftaroline to treat pseudomonas aeruginosa
NOOOOOOO
cefiderocol clinical uses
use limited to infections caused by resistant Gram-negative bacteria (ESBL, AmpC, or carbapenemases), but current place in therapy is still being determined
cephalosporin hypersensitivity
5 to 15% cross-reactivity with penicillins (esp 1st generation)
Can you try cephalosporin with a rash/itching to penicllin
PK to try
which cephalosporins have MTT side chain and why is it a problem
- cefamandole, cefotetan, cefmetazole, cefoperazone, moxalactam
- hypoprothrombinemia- due to reduction in vitamin K-producing bacteria in GI tract
- Ethanol intolerance
cephalosporins other adverse effects
- IV calcium and ceftriaxone precipitate, nonconvulsive statis epilepticus
true/false: a patient who developed anaphylaxis to penicllin can safely receive any cephalosporin
FALSE: a patient who developed anaphylaxis to penicllin may develop anaphylaxis to a cephalosporin (esp 1st gen) due to common nucleus/side chains. Therefore, skin testing and/or desensitization may be necessary before using a cephalosporin in this patient
what are the four commericial carbapenems
imipenem, meropenem, ertapenem, doripenem
carbapenem basic structure and what does it result in
- beta lactam ring attached to a 5-membered ring like the penicillins
- structural changes result in extended spectrum of activity and greater beta lactamase stability
carbapenem mechanism of action
inhibitors of cell wall synthesis by binding to and inhibting PBPs; primary target is PBP-2
are carbenems bactericidal? If so, in what manner?
Bactericidal in time-dependent manner
carbapenems mechanisms of resistance
- beta lactamase production
- decreased permeability
- alteration in PBPs
carbapenems spectrum of activity
have activity against gram-positive and gram-negative aerobes AND anaerobes
what specific gram positive aerobe does carbapenems have activity against
enterococcus faecalis
do carbapenems have activity against pseudomonas aeruginosa?
Yes, all of them except ertapenem
are carbapenems active against c. difficile
no
what gram negative anaerobe is carbapenem active against
bacteroides spp


























