Pharmacology Flashcards
(165 cards)
What are the 3 princpiple voltage and time dependent conductances in the heart?
• Voltage active sodium channels (depolarise) • Voltage activated calcium channels (depolarise) • Voltage activated potassium channels (repolarise)
What fundamentally differs nodal tissue for cardiac muscle tissue?
Pacemaker potential - diastolic potential/phase 4 slope (rather than horizontal line in cardiac muscle tissue at phase 4 - resting potential). Also atrial/ventricular cells have a plateau on the AP which nodal cells do not.
What is over-drive suppression?
SA node discharges APs at the highest rate and overrides the spontaneous discharge in the AV node and purkinje system and so is the dominant pacemaker
**What 4 conductances underlie the diastolic/phase 4 potential in pacemaker nodal cells?
1) Background sodium current (Ib) - leaky and present all of the time 2) Funny current (If) - hyperpolarisation mediated 3) Delayed rectifier potassium current (Ik) - switched off in phase 4, therefore contribute to depolarisation by inhibiting hyper polarisation) 4) Transient Voltage Activated Calcium Conductor (Icat) - kicks in at the end of phase 4 to give final kick to threshold => these all contribute to depolarisation and allow pacemaker cells to reach threshold

**Once threshold has been reached, which channel kicks in to bring the upstroke in nodal cells?
5) Long Calcium Channel (Ical) - inward movement of calcium underlies nodal AP (unlike in atrial/ventricular cells where it is sodium mediated)

**Which channels underlie repolarisation in nodal cells?
Delayed rectifier potassium current (Ik) which were previously switched off, now switch on and cause the outward flow of potassium

Why is there not a gradual depolarising drift in atrial/ventricular cells, like there is in nodal cells?
Because there is a constant trickling of potassium out of the cell by Ik1 - an inward rectifying conductor which helps maintain the resting membrane potential with slight hyper polarisation
What therefore causes an AP in atrial/ventricular myocytes, if not a gradual depolarising drift?
The arrival of depolarising influence from an adjacent cardiac muscle cell.
**What causes Phase 0: Depolarisation in atrial/ventricular myocytes?
Arrival of a depolarising AP from adjacent cardiac muscle cells causes voltage-activated sodium channels- INa channels to open and allow a large current of Na influx, activating rapidly.

**What causes Phase 1: Early repolarisation in atrial/ventricular myocytes?
Rapid opening/closing of transient outward K current – It0, which combined with INa channel closure results in a slight repolarisation. However, don’t get complete repolarisation, as long calcium currents – ICaL are now activated after a delay – this helps maintain the depolarisation

**What causes Phase 2: Plateau phase in atrial/ventricular myocytes?
Ca2+ channels have opened, ICaL. This maintains the plateau. At the same time there is a slowly developing delayed rectifying K efflux (outward) current (Ik). Fine balance between Ca influx and K efflux maintains the plateau. INaL channels (previous Na channels from Phase 0 that stayed open, and reverse §NCX1 channels contribute too)

**What causes Phase 3: Late repolarisation phase in atrial/ventricular myocytes?
Eventually the outward potassium (Ik) wins, and depolarisation occurs. This involves components IK channels which activate rapidly and IK1 channels which activate slowly

Which GPCRs do B1 adrenoreceptors preferably couple with?
Gs - which increases the conversion of ATP to cAMP, which mediates most of sympathetic activity of the heart
How does sympathetic innervation increase heart rate?
1) Increase the slope of diastolic phase 4 depolarisation (enhanced If and Ica activity) 2) Reduction of the threshold for AP initiation (enhanced Ica)
How does sympathetic innervation increase contractility?
1) increase in phase 2 plateau of the cardiac AP in atrial and ventricular myocytes, therefore enhanced Ca2+ influx 2) increased sensitisation of contractile proteins to Ca2+
What effect does sympathetic innervation have on the heart?
1) Increased HR 2) Increased contractility 3) Increased conduction velocity (smaller AV nodal delay to match HR) 4) Increased automaticity 5) Decreased duration of systole (to allow complete emptying of ventricles with new HR) 6) Decreased cardiac efficiency (oxygen consumption increases) 7) Increased activity of Na+/K+-ATPase 8) Increased mass of cardiac muscle
Which parasympathetic receptors lie in the heart and with GPCR do they coupe with?
M2 muscarinic choliceptors. Couple with Gi
What does parasympathetic stimulation do in the heart, and what effect does this have?
1) decreases activity of adenylate cyclase and reduces [cAMP]I 2) opens potassium channels This causes: - Decreased HR - Decreased contractility - Decreased conduction velocity in AV node
What are the 2 main vagal manoeuvres (which employ the parasympathetic system to suppress AV conduction)?
Valsalva manœuvre and massage of the bifurcation of the carotids
Which type of channels mediate the funny current?
Hyperpolarization-activated cyclic nucleotide gated (HCN) channels (HCN4 in heart).
What is ivabridine?
Blocker of the HCN (funny current) channel, and therefore decreases the pacemaker slope and reduces the HR. Used in angina in particular
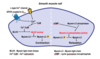
How does contraction occur in excitation-contraction couple in cardiac muscle?
- In the ventricular AP, we get Ca2+ entry during phase 2, due to voltage-activated Ca2+ channels (L-type) which moves into the cytoplasm 2. This creates a modest increase in the intracellular Ca2+ concentration, though this signal is not enough to cause an AP in itself, so needs to be amplified. 3. It does this using Calcium Induced Calcium Release (CICR) - The Ca2+ that has come through the L-type channels, bind to ryanodine-type 3 channel in the membrane of the SR, which is the main calcium store in the myocyte, and allow the efflux of calcium from the SR into the cytoplasm 4. This amplification leads to Ca2+ binding to troponin-c, which undergoes a conformational change which allows it to move tropmyocin from the synaptic cleft 5. This then allows cross-bridge formation between actin and myosin, resulting in contraction.
How does relaxation occur in excitation-contraction couple in cardiac muscle?
- Relaxation is controlled by the repolarisation in phase 3 to phase 4 of the AP 2. Repolarisation causes the voltage-activated Ca2+ channels to close 3. Ca2+ influx and CICR ceases and is pumped back to the sarcoplasmic reticulum, to be re-stored, by action of Ca2+ ATPase in the membrane of the SR. 4. Simultaneously, Na+/Ca2+ exchanger (NCX) pushes 1Ca out and 3Na in. 5. Under these 2 mechanisms, Ca2+ dissociates from troponin-C; the cross-bridges break up; actin and myosin are pumped back into the synaptic cleft and we get relaxation.
What are examples of β-adrenoceptor agonists?
Dobutamine, adrenaline and noradrenaline (catecholamines)