W10 Carbohydrates Flashcards
(14 cards)
Define carbohydrates
- Polyhydroxyl aldehydes and ketones
- General formula approx. Cn(H2O)m
- Energy storage material that allows plants to convert sunlight to energy
Nomenclature of carbohydrates
- Aldehyde or ketone (aldo or keto)
- Number of carbons (tri-, tetra-, penta-)
- If saccharide, will have -ose as a suffix
- D- or L- depending on stereochemistry of highest numbered stereogenic carbon
Number of enantiomers that a single carbohydrate has
A single carbohydrate will have 2n isomers where n is the no. chiral centres
Fischer projections
and how to draw
- Allows us to determine if carbohydrate is D- or L-
-
Most oxidised carbon needs to be at the top
- Aldehyde
- Configurational centre is highest numbered chiral centre (with counting starting from oxidised end)
-
Draw a cross of groups around the central carbon
- Horizontal line represents bonds coming out of page (will generally be H and OH)
- Vertical line represents bonds going into page
Note: might need to do a rotation around C-C bond to get the required orientation
- D- have hydroxyl to the right of highest numbered chiral centre (2ND LAST CARBON)
L- has to the left
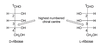
Cyclisation of aldoses
- Can undergo nucleophilic addition reaction with hydroxyl group of 5th carbon (highest numbered chiral centre) to form a 6 membered cyclic hemiacetal
- Creates a new stereocentre: the anomeric centre
- α = axial anomeric centre (down on Haworth projection)
- β = equatorial anomeric centre (up on Haworth projection)
- Note that for cyclic glucose (called D-glucopyranose) all hydroxyls are equatorial -> can use to figure out positions for other sugars
Using rings to name cyclic carbohydrates
- hexagonal ring: [carbo]pyran[ose]
- pentagonal ring: [carbo]furan[ose]
Both have O in the cyclic disk and double bonds
Mutarotation
Occurs when two anomers are possible (because anomeric centre has a hydroxyl attached)
Each anomer has its own optical rotation
This leads to a change in optical rotation with time -> mutarotation

Haworth projections
Molecule is squished so constituents of ring are either pointing up or down
(conformational isomerisation is not displayed
- α anomers have anomeric hydroxyl down (axially)
- β anonmers have anomeric hydoxyl pointing up (equatorially)
For glucose
- All OHs are in equatorial position
- Except for anomeric carbon which could be axial or equatorial
- Can use to decide positions of other isomers of glucose (see what side has switched in fisher projection)
Reduction of carbohydrates
Converts carbonyl group to alcohol
Requires NaBH4/LiAlH4 or H2 and catalyst
As ring forms are in equilibrium with open-chain forms, they too are readily reduced
Oxidation of carbohydriates - practical purpose
Oxidation of carbohydate is used as a test for the presence of an aldehyde or ketone group
primary alcohol -> aldehyde
secondary alcohol -> ketone
Reducing sugars
- Sugars that can oxidise
- MUST have a hydroxyl on anomeric carbon (1st carbon) -> means they can mutarotate
- Aldehyde oxidises to a carboxylic acid (in presence of oxidising agent)
- Tautomers (enol forms) of ketone allow it to oxidise to carboxylic acid by first becoming an aldehyde

What are glycosides
How are they formed
How can we test for glycosides
Glycosides are acetals of carbohydrates
Cyclic form of carbohydrate (hemiacetal) is converted to acetal using alchol and acid catalyst
Acetals don’t mutarotate -> aren’t reducing sugars -> Don’t react with Tollen’s reagent or Fehling’s solution
Formation of hemiacetal from glycoside



