Chapter 13 Flashcards
(28 cards)
What is the citric acid cycle?
in mitochondria
won’t occur anaerobically becuase NADH and FADH2 will accumulate if oxygen isn’t available for ETC
function= oxidation of acetyl-CoA to carbon dioxide
energy released from oxidation is saved as NADH, FADH2 and GTP

Where are the enzymes of the TCA?
in the matrix of the mitochondria
except succinate dehydrogenase–in in the inner membrane
–also functions as complex II in the ETC
What inhibits and activates isocitrate dehydrogenase?
major control enzyme
inhibited by NADH and ATP
activated by ADP
What does alpha ketoglutarate dehydrogenase require?
thiamine, lipoic acid, CoA, FAD, and NAD
What is the function of succinyl CoA synthase (succinate thiokinase)?
catalyzes substrate level phosphorylation of GDP to GTP
What is the function of citrate synthase?
condenses incoming acetyl group with oxaloacetate to form citrate
What are the steps of the citric acid cycle?
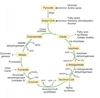
What intermediates of the cycle have other functions?
1) citrate may leave mitochondria (citrate shuttle) to deliver acetyl CoA into the cytoplasm for fatty acid synthesis
2) succinyl CoA is high energy intermediate; used for heme synthesis and to activate ketone bodies in extrahepatic tissues
3) malate can leave mitchondria (malate shuttle) for gluconeogensis
How are electrons transferred from NADH and FADH2
NADH: produced in cytoplasm and either malate shuttle or alpha glycerol phosphate shuttle can transfer electrons in mitochondria for deliverty to ETC
FADH2: produced by succinate dehydrogenase in TCA and alpha-glycerol phosphate shuttle
–enzymes located in inner membrane and can reoxidize FADH2 directly by transferring electrons into ETC
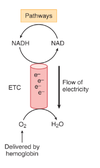
What is the function of oxygen in the ETC?
O2 is delivered to tissues by hemoglobin
–majority of oxygen required in tissue is consumed in ETC
accepts electrons atthe end of chain and forms water
What are the steps of oxidative phosphorylation?

What are the steps of the electron transport chain?
1) NADH dehydrogenase (complex I) accepts electrons from NADH
2) coenyzme Q (lipid)
3) cytochrome b/c1 (Fe/heme protein; complex III)
4) cytochrome c (Fe/heme protein)
5) cytochrome a/a3 (Cu/heme protein; cytochrome oxidase, complex IV transfers electrons to oxygen
–all components in inner membrane of mitochondria
–succinate dehydrogenase and alpha glycerol phosphate shuttle enzymes reoxidize their FADH2 and pass electrons directly to CoQ
How is the protein gradient generated?
-electricity generated by ETC used to run proton pumps–drive portons from matrix space across inner membrane and into intermembrane space–create pH gradient
–complexes I, III, IV translocate protons
proton gradient is normally maintained across mitochondrial inner membrane
–if proton channels open, protons run back into matrix—part of oxidative phosphorylation complex
How does oxidative phosphorylation occur?
ATP syntehsis uses energy of proton gradient
carried out by F0F1 ATP synthase complex
–spans inner membrane
protons flow into mitochodnria through F0 component, and their energy is used by F1 component (ATP synthase) to phosphorylate ADP using Pi
How can you predict a myocardial infarction?
troponin I and troponin T are sensitive and specific markers
–appear 3-6 hours after onset of symptoms, peak by 16 hours, and remain elevated fo a week
LDH isozyme analysis: LDH1>LDH2 peaks 2-3 days following an MI
–useful if patient report chest pain that occured several days before
What is tissue hypoxia?
deprives ETC of sufficient oxygen, decreasing rate of ETC and ATP production
–when ATP levels falls, glycolysis increases and in absence of oxygen will produce lactate (lactic acidosis)
–anaerobic glycolysis can’t meet demand of most tissues for ATP especially highly aerobic tissues (cardiac, nerves)
What occurs in a myocardial infarction?
mocytes swell as membrane potential collapses and cell gets leaky
enzymes released from damaged tissue and lactic acidosis contributes to protein precipitation and coagulation necrosis
What do inhibitors of the ETC do?
effectively inhibit the whole coupled process
result in: decreased oxygen consumption, increased intracellular NADH/NAD and FADH2/FAD ratios, decreased ATP
ex. cyanide and carbon monoxide
antimycin (cytochrome b/c1), doxorubicin (CoQ), oligomycin (F0)
What is the effect of cyanide on the ETC?
binds irreversibly to cytochrome a/a3, preventing electron transfer to oxygen
sources: burning polyurethane, byproduct of nitroprusside
treament: nitrates
–convert hemoglobin to methemoglobin which binds cyanide in the blood before it reaches the tissues
What is the effect of carbon monoxide on the ETC?
binds to cytochrome a/a3 –less tightly than cyanide
–binds to hemoglobin displacing oxygen
SYMPTOMS: headache, nausea, tachycardia, tachypnea, cherry red lips and cheeks, respiratory depression and coma
sources: propane heaters, gas grills, vehicle exhaust, tobacco smoke, house fires, methylene chloride-based paint strippers
What is the effect of uncouplers?
decrease proton gradient
–decreased ATP synthesis, increased oxygen consumption, increased oxidation of NADH
–rate of ETC increases with no ATP synthesis so energy released as heat
ex. 2,4 dinitrophenol (2,4 DNP) and aspirin (other salicylates), UCP, thermogenin (brown adipose tissue–energy loss as heat to maintain a basal temperature around kidneys, neck, breastplate and scapulae in newborns)
What occurs with aspirin to treat RA?
uncoupling of oxidative phosphorylation, increased O2 consumption, depletion of hepatic glycogen, and pyretic effect oftoxic doses of salicylate
–salicylate intoxication can cause tinnitus to pronounced CNS and acid-base disturbances
What are reactive oxygen species?
parially reduced molecular oxygen
–react rapidly iwth lipid to cause peroxidation with proteins and other substrates; resutling in denaturation and preciptation in tissues
ex. superoxide, hydrogen peroxide, hydroxyl radical
What destroys ROS in cells?
small quanitties of ROS are inevitable by products of ETC in mitochondria
–normally destroyed by catalase and superoxide dismutase


