Chem II: 6-10 Flashcards
(184 cards)
lewis acid
electron acceptor
tend to be electrophiles
vacant p orbitals that can accept electron pair
lewis base
electron donor
tend to be nucleophiles
have lone pair of electrons that can be donated
coordinate covalent bonds
covalent bonds in which both electrons in the bond came from the same starting atom (lewis base)
when lewis acid and bases interact
amphoteric
molecules that can act as either bronsted lowry acids or bases
ex: water
acid dissociatio constant
Ka
measures strength of an acid in solution
dissociation of acid HA
HA = H+ + A-
pKa of more acid molecules
smaller or negative pKa
pKa of more basic molecules
larger pKa
main functional groups that act as acids
alcohols, aldehydes and ketones (at alpha carbon), carboxylic acids and most derivatives
main functional groups that act as bases
amines and amides
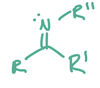
nucleophiles
have either lone pairs or pi bonds that can form new bonds to electrophiles
tend to be good bases
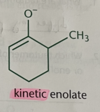
strength based on rate of rxn - kinetic property
the more basic the nucleophile, the more ___ it is
reactive
factors that contribute to nucleophilicity
- charge: inc with inc electron density (more neg charge)
- electronegativity: dec as electroneg inc (bc less likely to share electron density)
- steric hindrance: dec as molecule is bulkier
- solvent: protoic solvents hinder
electrophile
- electron loving species with a positive charge or positively polarized atom
- accepts an electron pair when forming new bonds with a nucleophile
- usually act as lewis acids
- better leaving groups make it more likely that a rxn will happen
electrophilicity of carboxilic acid derivatives
most reactive to least: anhydrides, carboxilic acids, esters, amides
leaving groups
- retain the electrons after heterolysis
- stabilize extra electrons
- weak bases and conjugate bases of strong acids are GLGs
heterolytic rxns
- opposite of coordinate covalent bond formation
- a bond is broken and both electrons are given to one of the 2 products
nucleophilic subsitution reactions
SN1 and SN2
nucleophile forms a bond with a substrate carbon and LG leaves
SN1 steps
- LG leaves, making a carbocation: rate limiting step
- nucleophile attacks carbocation

SN1
- first order reaction
- loss of leaving group to make carbocation intermediate then nucl attack
- produce usually racemic mixture
- varied sterochemsitry bc nucl can attack C+ from either side
- most likely to occur on tertiary carbons (C+ easily stabilized)
SN2
- one step -> concerted rxn
- backside attack
- inversion
- stereospecific reaction
- 1° and 2° NOT 3° carbons
stereospecific reaction
configuration of reactant determines the configuration of the product due to the reaction mechanism
SN2 steps

how must the nucleophile and LG be related in order for a substitution reaction to proceed?
a substitution reaction will proceed when the nucleophile is a stronger base (more reactive) than the LG