Physics I: 6-8 Flashcards
(169 cards)
fluids
substances that have the ability to flow and conform to the shapes of their containers
fluids can exert ____ forces, but cannot exert ____ forces
perpendicular
shear
shear forces
tangential forces
density
ρ
mass per unit volume of a substance (fluid or solid)
scalar
density eq
ρ = m/V
density of water
1 g/cm3 = 1000 kg/m3
weight in terms of density eq
Fg = ρVg
V = volume
specific gravity eq
ρ / 1 g/cm3
pressure
P
ratio of the force per unit area
scalar
pressure in terms of force eq
P = F/A
F = magnitude of normal force
SI unit
pressure
pascal Pa
why is pressure scalar rather than vector?
pressure is the same at all points along the walls of its container and within the space of the container itself
pressure applies in all directions at any point
atmospheric pressure
changes with altitude
pressure exerted by a gas against the walls of its container will always be ________ to the container walls
perpendicular (normal)
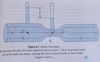
absolute (hydrostatic) pressure
total pressure exerted on an object that is submerged in a fluid
sum of all pressures at a certain point within a fluid
absolute pressure eq
P = P0 + ρgz
P0 = incident or ambient pressure
z = depth of object
gauge pressure
difference between absolute pressure and atmospheric pressure
amount of pressure in a closed space above and beyond atmospheric pressure
gauge pressure eq
Pgauge = P - Patm = (P0 + ρgz) - Patm
when does the gauge pressure equal the fluid pressure?
when atmospheric pressure is the only pressure above the fluid column
hydrostatics
study of fluids at rest and the forces and pressures associated with standing fluids
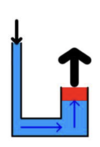
pascal’s principle
a pressure applied to an incompressible fluid will be distributed undiminished throughout the entire volume of the liquid
hydraulic machines
operate based on the application of pascal’s principle to generate mechanical advantage
generate output force by magnifying an input force by a factor equal to the ratio of the cross sectional area of the larger piton to that of the smaller piston

hydraulic lift eqs

according to pascal’s principle, the larger the area, the ___ the force…
larger
although this force will be exerted through a smaller distance