Unit 4 Flashcards
(32 cards)
Why would we need proteases?
- Protein turnover
- Maintain protein homeostasis (protein balance)
- Digestion (w/ food)
- Activation of inactivation of proteins
- Formulation of multiple polypeptides from one polypeptide chain
How do proteases cleave peptides?
By hydrolysis (addition of water)
Why are peptide bonds stable?
Resonance structures reduces +ve charge on carboxyl, which reduces reactivity
What is chymotrypsin and where can it be found?
A serine protease in small intestines
What types of amino acids does chymotripsin cleave?
Large hydrophobic AA’s, so Trp, Phe, Met, Tyr
How is chymotrypsin syntehsized?
Synthesized as cymotrypsinogen in pancreas
Activated by trypsin in small intestines to form pi-chymotrypsin, which then autolyses to form a-chymotrypsin (active)

What is the location of the conserved serine nucleophile?
AA 195 (Ser195)
What is the general reaction of chymotrypsin?
Ser195 attacks acyl group of peptide bond, cleaving the peptide bond and forming an intermediate. Then, the intermediate is cleaved by water to release the carboxyl group.

What is the catalytic triad contained within chymotrypsin?
Asp 102, His 57, and Ser 195

How does the catalytic triad increase Ser 195’s reactivity as a nucleophile?
Asp 102 H-bonds with imidazole ring on His 57, making His 57 slightly positive (proton acceptor)
His 57 also H-bonds with Ser 195; His57 has acidic character and acts as a BASE CATALYST (catalyzing Ser 195 basicity)
Ser 195 thus becomes polarized from His 57 H-bonding

What is step 1 of chymotrypsin catalysis?
Substrate binds to active site; hydrophobic AA in hydrophobic pocket near active site
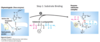
What is step 2 of chymotrypsin catalysis?
Alkoxide ion on Ser 195 (formed due to His 57 base catalysis) performs nucleophilic attack on acyl carbon, forming a transient unstable tetrahedral confirmation stabelized by Gly 195 (H bond stabelizes oxyanion tetrahedral)

What is step 3 of chymotrypsin catalysis?
Tetrahedral oxyanion tetrahedral collapses, reforming carbonyl and breaking peptide bond. His 57 acts as acid catalyst, donating H to the leaving amino peptide fragment. Remaining carbonyl (C=O) stabilized by Ser 195

What is step 4 of chymotrypsin catalysis?
Water enters active site and gains hydroxide-like character due to His 57 base catalysis

What is step 5 of chymotrypsin catalysis?
Water’s partialy negative oxygen acts as a nucleophile (with His 57 acting as a base catalyst) forming a tetrahedral oxyanion (again stabelized by Gly 193 H-bond with oxyaninon)

What is step 6 of chymotrypsin catalysis?
Tetrahedral oxyanion rearranges to form carbonyl (in carboxylic acid) ad free Ser 195. His 57 acts as acid catalyst to assist in this process.

What is step 7 of chymotrypsin catalysis?
“New” carbonyl peptide leaves active site, allowing cycle to restart.

What are similarities and differences between chymotrypsin, trypsin, and elastase?
Similarities: They are all serine proteases, utilizing the Serine-Histidine-Aspartate catalytic triad
Differences: Active sites are different & thus cleave different AA peptide bonds
Chymotrypsin has a large hydrophobic pocket for large hydrophobic sidechains
Trypsin has Asp at the bottom of the pocket for +vely charged AA (since + & - charge matches)
Elastase has two Valine residues that narrow down the pocket to alow the binding of small hydrophobic R groups (ex. Val, Ala)

What are cysteine proteases? Provide an example.
Proteases that use cysteines as a nucleophile
E.g. caspases involved in apotosis
What are aspartate proteases? Provide an example.
Ues 2 aspartate residues to act as base catalysts, such that H2O can be used as a strong nucleophile
E.g. HIV protease

What are metalloproteases? Provide an example.
Proteases that use metal ions to make water into a strong nucleophile
E.g. MMP, Matrix Metalo Protease (in cellular matrix)
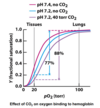
What is Hemoglobin, what is it used by, and what does it do?
Hemoglobin (Hb) is a protein used to carry oxygen. Used by RBC. Carries O2 from lungs (pH=7.4) to tissue (pH=7.2)
What is the structure of Hemoglobin? Include the amino acids contained in each, as well as the secondary structures that it is made up of.
2x α and 2x ß subunits
α subunits have 144 AA and 7 helixes
ß have 146 AA and 8 helixes
Each subunit has one heme group (4 in total)

How are Hemoglobin subunits bound together? Which are strong interactions, and which are weak?